Question
2. Nickel-cadmium batteries employ the following reaction: Cd (s) + NiO(OH) (s) Cd(OH)2 (s) + Ni(OH)2 (s) a) Balance this redox reaction in basic
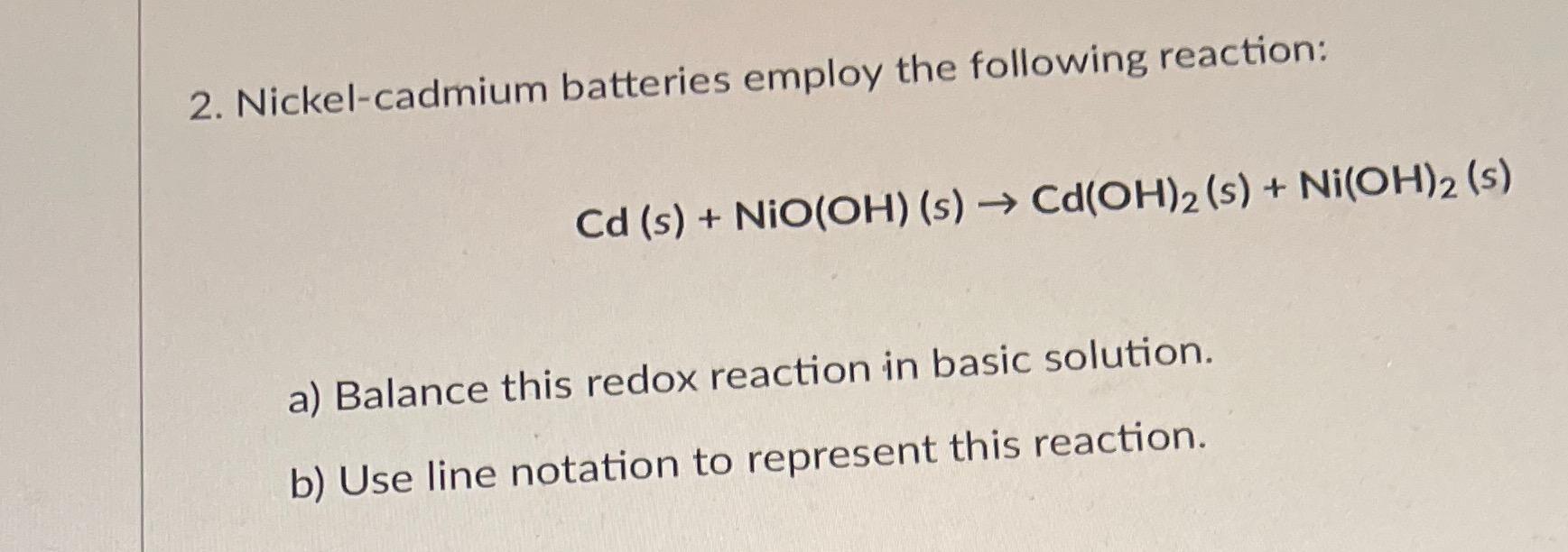
2. Nickel-cadmium batteries employ the following reaction: Cd (s) + NiO(OH) (s) Cd(OH)2 (s) + Ni(OH)2 (s) a) Balance this redox reaction in basic solution. b) Use line notation to represent this reaction.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Business Law and the Legal Environment
Authors: Susan S. Samuelson, Jeffrey F. Beatty
7th edition
1285860381, 978-1305445864, 1305445864, 978-0357689646, 978-1285860381
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



