Question
2. Styrene is polymerized by free radical mechanism in solution. The initial monomer and initiator concentrations are 1 M and 1 mM, respectively. At the
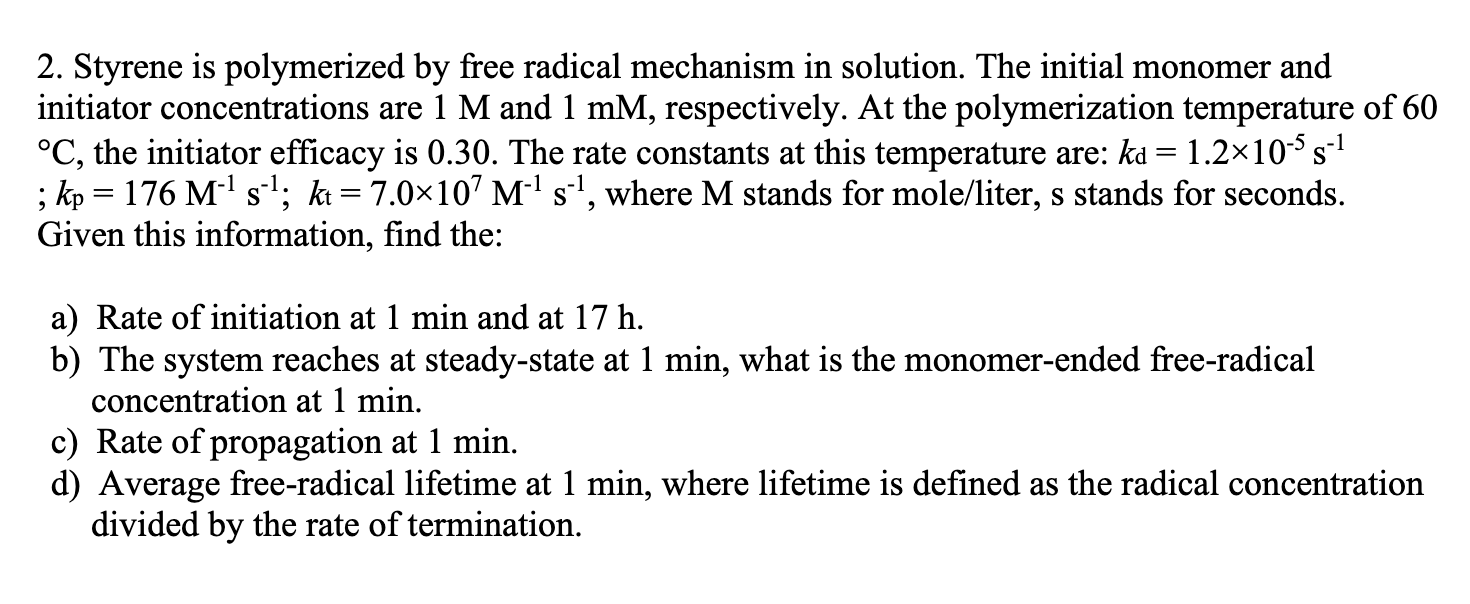
2. Styrene is polymerized by free radical mechanism in solution. The initial monomer and initiator concentrations are 1 M and 1 mM, respectively. At the polymerization temperature of 60 C, the initiator efficacy is 0.30. The rate constants at this temperature are: kd = 1.210-5 s-1 ; kp = 176 M-1 s-1; kt = 7.0107 M-1 s-1, where M stands for mole/liter, s stands for seconds. Given this information, find the: a) Rate of initiation at 1 min and at 17 h. b) The system reaches at steady-state at 1 min, what is the monomer-ended free-radical concentration at 1 min. c) Rate of propagation at 1 min. d) Average free-radical lifetime at 1 min, where lifetime is defined as the radical concentration divided by the rate of termination.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


