Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. Under conditions of strong pore diffusion the reaction AR proceeds at 700C on a slowly deactivating catalyst by a first-order rate rA=0.03CA(mole/gcat.min) Deactivation is
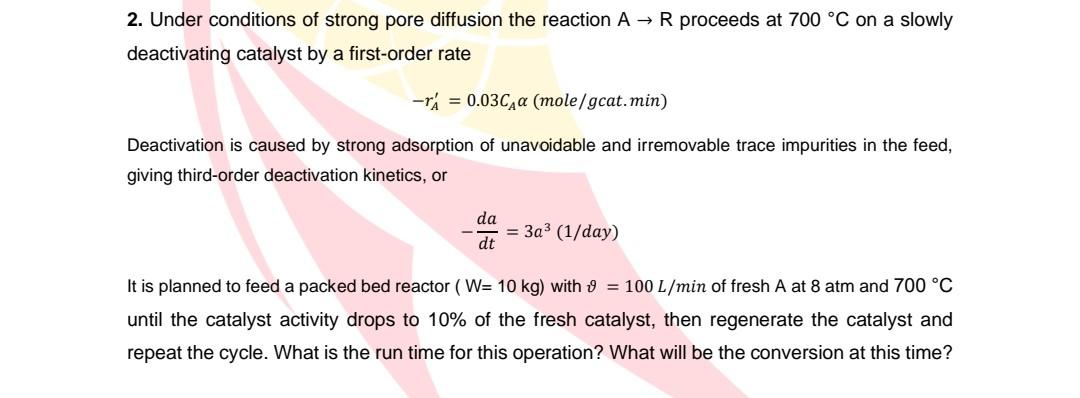
2. Under conditions of strong pore diffusion the reaction AR proceeds at 700C on a slowly deactivating catalyst by a first-order rate rA=0.03CA(mole/gcat.min) Deactivation is caused by strong adsorption of unavoidable and irremovable trace impurities in the feed, giving third-order deactivation kinetics, or dtda=3a3(1/day) It is planned to feed a packed bed reactor ( W=10kg ) with =100L/min of fresh A at 8atm and 700C until the catalyst activity drops to 10% of the fresh catalyst, then regenerate the catalyst and repeat the cycle. What is the run time for this operation? What will be the conversion at this time
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


