Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(2) We have a following reaction which is taking place in a semi-batch reactor. A+BC+D+E(g) A 1.5-molar solution of A is fed at a rate
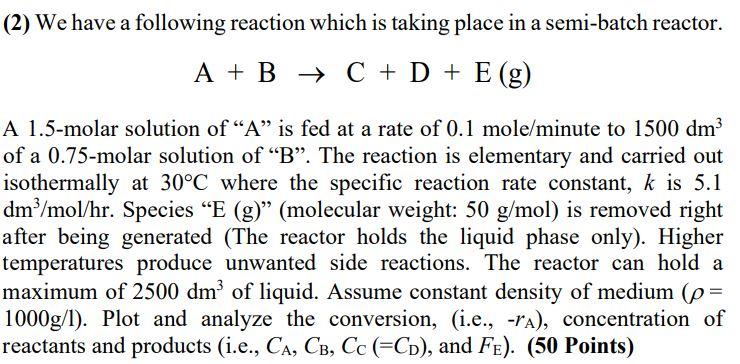
(2) We have a following reaction which is taking place in a semi-batch reactor. A+BC+D+E(g) A 1.5-molar solution of "A" is fed at a rate of 0.1 mole/minute to 1500dm3 of a 0.75-molar solution of "B". The reaction is elementary and carried out isothermally at 30C where the specific reaction rate constant, k is 5.1 dm3/mol/hr. Species "E (g) " (molecular weight: 50g/mol ) is removed right after being generated (The reactor holds the liquid phase only). Higher temperatures produce unwanted side reactions. The reactor can hold a maximum of 2500dm3 of liquid. Assume constant density of medium ( = 1000g/l ). Plot and analyze the conversion, (i.e., rA ), concentration of reactants and products (i.e., CA,CB,CC(=CD), and FE). (50 Points)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


