Question: (20%) Problem 5: Hot air balloons float by heating the air inside an inflatable compartment. Consider such a balloon which has an inflatable compartment
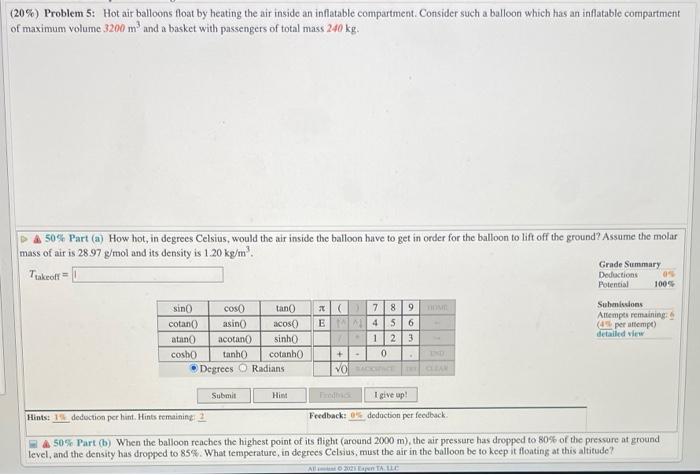
(20%) Problem 5: Hot air balloons float by heating the air inside an inflatable compartment. Consider such a balloon which has an inflatable compartment of maximum volume 3200 m and a basket with passengers of total mass 240 kg. 50% Part (a) How hot, in degrees Celsius, would the air inside the balloon have to get in order for the balloon to lift off the ground? Assume the molar mass of air is 28.97 g/mol and its density is 1.20 kg/m. Ttakeoff sin() cotan() atan() cosh()) cos() asin() acotan() tanh() Degrees Submit tan() X ( acos() E sinh() cotanh() Radians 7 8 9 4 5 6 12 3 0 VO SACOCE CLEAN + . Hint . DOME I give up! Feedback: 0% deduction per feedback. Grade Summary Deductions Potential 0% 100% Submissions Attempts remaining: (4% per attempt) detailed view Hints: 1% deduction per hint. Hints remaining: 2 &50% Part (b) When the balloon reaches the highest point of its flight (around 2000 m), the air pressure has dropped to 80% of the pressure at ground level, and the density has dropped to 85%. What temperature, in degrees Celsius, must the air in the balloon be to keep it floating at this altitude? All 2021 Espen TA. LLC
Step by Step Solution
3.51 Rating (161 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


