Answered step by step
Verified Expert Solution
Question
1 Approved Answer
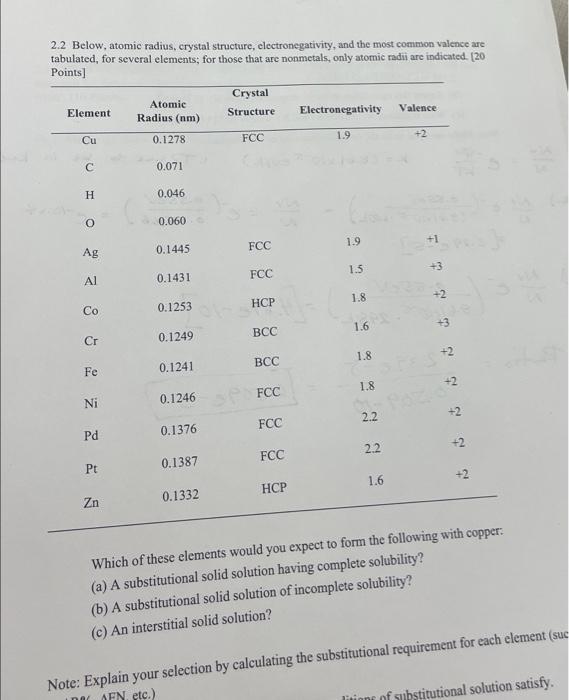
2.2 Below, atomic radius, crystal structure, electronegativity, and the most common valence are tabulated, for several elements; for those that are nonmetals, only atomic radii
2.2 Below, atomic radius, crystal structure, electronegativity, and the most common valence are tabulated, for several elements; for those that are nonmetals, only atomic radii are indicated. [20 Points] Element Cu C H O 20 Z 8 5 2 2 Ag Co Cr Ni Pd Pt Zn Atomic Radius (nm) 0.1278 RO 0.071 0.046 0.060 0.1445 0.1431 0.1253 0.1249 0.1241 0.1376 0.1387 Crystal Structure 0.1332 FCC FCC FCC HCP 0.1246 SPO FCC BCC BCC FCC FCC HCP Electronegativity 1.9 1.9 1.5 1.8 1.6 1.8 1.8 2.2 2.2 Valence 1.6 +2 523 +1 +3 SPES Pozo +2 +3 +2 +2 +2 +2 +2 3 J5 Which of these elements would you expect to form the following with copper: (a) A substitutional solid solution having complete solubility? (b) A substitutional solid solution of incomplete solubility? (c) An interstitial solid solution? 5/5 3/3 ottust Note: Explain your selection by calculating the substitutional requirement for each element (suc AFN, etc.) ditions of substitutional solution satisfy.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


