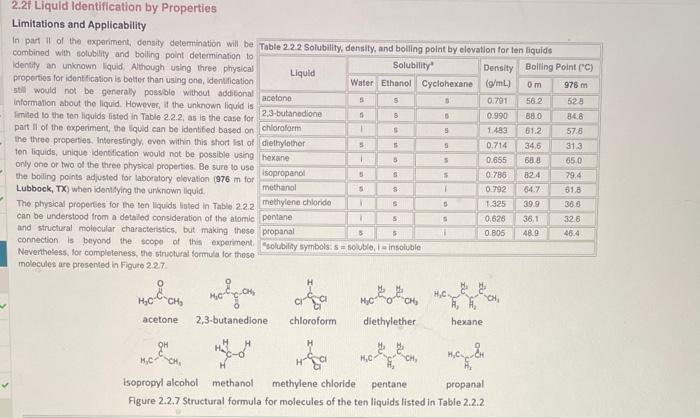
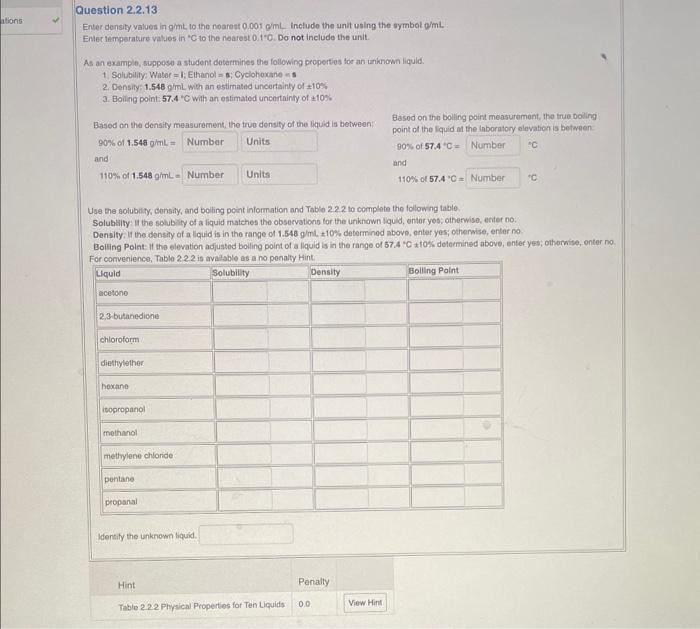
2.2. Liquid Identification by Properties Limitations and Applicability In part II of the exporiment, consity deterrination will be combined with solubility and boiling point determination to identity an uninown fiquid. Athough using three physical properties for identification is betier than using one, ldentification 5till would not be generaly possble without additonal informalion about the liquid. Howover, at the unknown liquid is Imited to the ten liquids listod in Table 2.2.2, as is the case for pat ll of the experiment, the Iquid can be idontifed based on the threo propertes. Interesingly, even within this short list of ten liquids, unique identication would not be possible using only one or two of the three physical properties. Be sure to use the boiling points adjusted for laboratory olovation (976m fol Lubbock, TX ) when identifying the unknown liquid. The physical properses for the ten liquids lioted in Table 2.2.2 can be understood trom a detaied consideration of the alomik and structural molocular characteristics. but making these connection is beyond the scopes of this experiment Neventheless, lor completeness, the structural formula lor these molecules are prosented in Figure 2.27. Use the solubily, consity, and boling point information and Table 2.2.2 to comphete the following table. Solubility it the solubity of a liquid matches the observations for the uniknawn iquid, entor yos: otherwise, enter no: Density. If the density of a llquid is in the range of 1.548g/mL+10% determined above, enter yes; chhensise, enter no. Bolling Polnt If the elevation adjusted boiling point of a lquid is in the range of 57.4C+ toks deleemined above, enter yes; otherwise, onter no. Fer onnunerianca. Table 2.22 is avalable as a no penalty Hint. 2.2. Liquid Identification by Properties Limitations and Applicability In part II of the exporiment, consity deterrination will be combined with solubility and boiling point determination to identity an uninown fiquid. Athough using three physical properties for identification is betier than using one, ldentification 5till would not be generaly possble without additonal informalion about the liquid. Howover, at the unknown liquid is Imited to the ten liquids listod in Table 2.2.2, as is the case for pat ll of the experiment, the Iquid can be idontifed based on the threo propertes. Interesingly, even within this short list of ten liquids, unique identication would not be possible using only one or two of the three physical properties. Be sure to use the boiling points adjusted for laboratory olovation (976m fol Lubbock, TX ) when identifying the unknown liquid. The physical properses for the ten liquids lioted in Table 2.2.2 can be understood trom a detaied consideration of the alomik and structural molocular characteristics. but making these connection is beyond the scopes of this experiment Neventheless, lor completeness, the structural formula lor these molecules are prosented in Figure 2.27. Use the solubily, consity, and boling point information and Table 2.2.2 to comphete the following table. Solubility it the solubity of a liquid matches the observations for the uniknawn iquid, entor yos: otherwise, enter no: Density. If the density of a llquid is in the range of 1.548g/mL+10% determined above, enter yes; chhensise, enter no. Bolling Polnt If the elevation adjusted boiling point of a lquid is in the range of 57.4C+ toks deleemined above, enter yes; otherwise, onter no. Fer onnunerianca. Table 2.22 is avalable as a no penalty Hint