Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2.4 Find outlet concentrations. 2.5 Find number of equilibrium. A solution of acetic acid (A) in water (C) is to be extracted using isopropyl ether
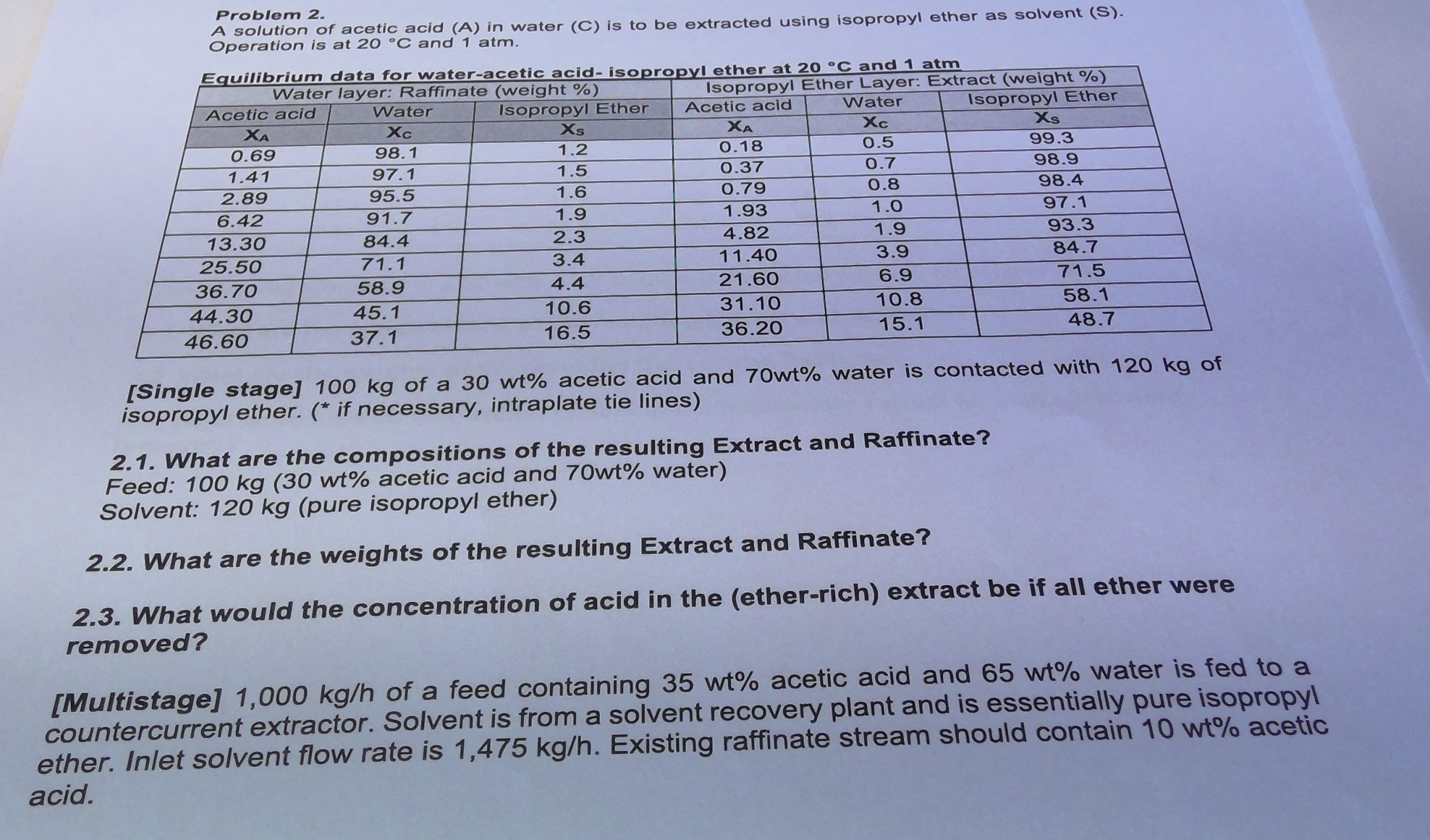
2.4 Find outlet concentrations.
2.5 Find number of equilibrium.
A solution of acetic acid (A) in water (C) is to be extracted using isopropyl ether as solvent (S). Problem 2. Operation is at 20C and 1atm. [Single stage] 100kg of a 30wt% acetic acid and 70wt% water is contacted with 120kg of isopropyl ether. (* if necessary, intraplate tie lines) 2.1. What are the compositions of the resulting Extract and Raffinate? Feed: 100kg (30 wt\% acetic acid and 70wt% water) Solvent: 120kg (pure isopropyl ether) 2.2. What are the weights of the resulting Extract and Raffinate? 2.3. What would the concentration of acid in the (ether-rich) extract be if all ether were removed? [Multistage] 1,000kg/h of a feed containing 35wt% acetic acid and 65wt% water is fed to a countercurrent extractor. Solvent is from a solvent recovery plant and is essentially pure isopropy ther. Inlet solvent flow rate is 1,475kg/h. Existing raffinate stream should contain 10wt% aceti cidStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


