Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2.8. Consider a closed vessel initially containing 1mol of pure ethyl acetate at 70C and 86kPa. Imagine that pure ethanol is slowly added at constant
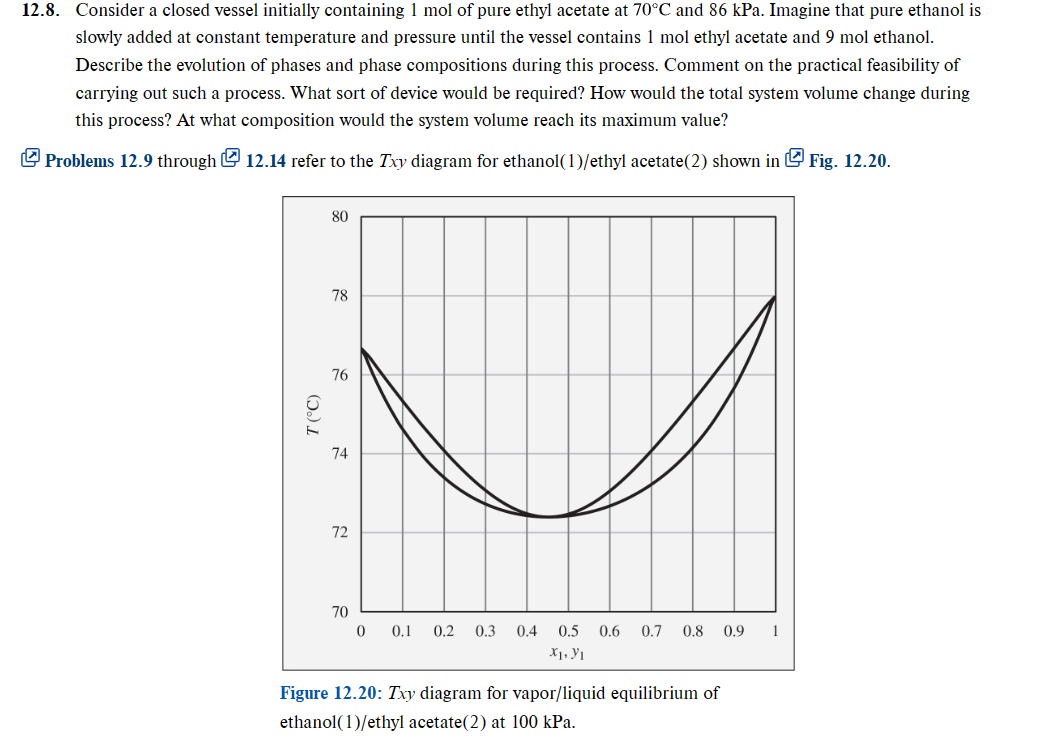
2.8. Consider a closed vessel initially containing 1mol of pure ethyl acetate at 70C and 86kPa. Imagine that pure ethanol is slowly added at constant temperature and pressure until the vessel contains 1mol ethyl acetate and 9mol ethanol. Describe the evolution of phases and phase compositions during this process. Comment on the practical feasibility of carrying out such a process. What sort of device would be required? How would the total system volume change during this process? At what composition would the system volume reach its maximum value? Problems 12.9 through 12.14 refer to the Txy diagram for ethanol(1)/ethyl acetate(2) shown in Fig. 12.20. Figure 12.20: Txy diagram for vapor/liquid equilibrium of ethanol(1)/ethyl acetate(2) at 100kPa
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


