Answered step by step
Verified Expert Solution
Question
1 Approved Answer
28.6 A horizontal chemical vapor deposition (CVD) reactor similar to the configuration shown in Example 3, Figure 28.6 will be used for growth of gallium
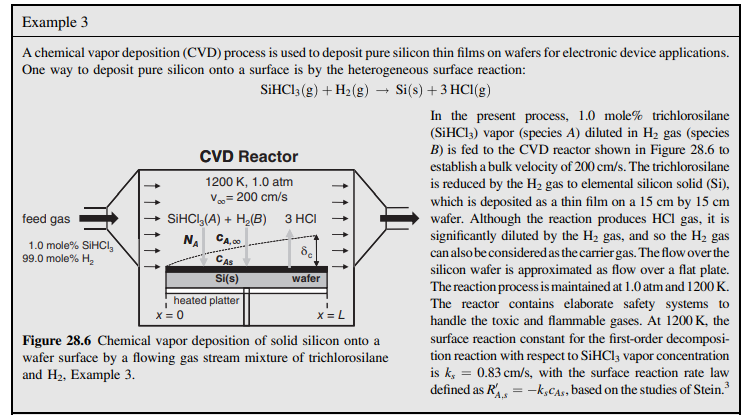 28.6 A horizontal chemical vapor deposition (CVD) reactor similar to the configuration shown in Example 3, Figure 28.6 will be used for growth of gallium arsenide (GaAs) thin films. In this process, arsine vapor, trimethylgallium vapor, and H2 gas are fed into the reactor. Inside the reactor, the silicon wafer rests on a heated plate called a susceptor. The reactant gases flow parallel to the surface of the wafer and deposit a GaAs thin film according to the simplified CVD reactions 2AsH3(g)2As(s)+3H2(g)2Ga(CH3)3(g)+3H2(g)2Ga(s)+6CH4(g) If the reactant gas is considerably diluted in H2 gas, then the mass transfer of each species in the H2 carrier gas can be treated separately. These surface reactions are considered to be very rapid, and so the mass transfer of the gaseous reactants to the surface of the wafer limits the rate of GaAs thin-film formation. In the present process, a 15cm15cm square silicon wafer is positioned at the leading edge of the susceptor plate. The process temperature is 800K, and the total system pressure 101.3kPa (1.0 atm). The feed gas delivered to the reactor results in a bulk linear velocity of 100cm/s. The composition of arsine and trimethylgallium in the feed gas are both 0.10 mole\%, which is very dilute. You may assume that the amount of arsine and trimethylgallium delivered with the feed gas is much higher than the amount of arsine and trimethylgallium consumed by the reactions, so that the concentration of these reactants in the bulk gas phase is essentially constant down the length of the reactor. You may also assume that the surface-reaction rates are instantaneous relative to the rates of mass transfer, so that the gasphase concentrations of both arsine vapor and trimethylgallium vapor at the surface of the wafer are essentially zero. The binary gas-phase diffusion coefficient of trimethylgallium in H2 is 1.55 cm2/s at 800K and 1.0atm. a. What are the average mass-transfer rates for arsine and trimethylgallium over the whole wafer? b. Based on the ratio of the arsine and trimethylgallium masstransfer rates, what is the composition of the GaAs composite thin film-e.g., the molar composition of gallium (Ga) and arsenic (As) in the solid? How could the feed-gas composition be adjusted so that the molar ratio of Ga to As within the solid thin film is 1:1 ? A chemical vapor deposition (CVD) process is used to deposit pure silicon thin films on wafers for electronic device applications. One way to deposit pure silicon onto a surface is by the heterogeneous surface reaction: SiHCl3(g)+H2(g)Si(s)+3HCl(g) In the present process, 1.0 mole\% trichlorosilane ( SiHCl3 ) vapor (species A ) diluted in H2 gas (species B ) is fed to the CVD reactor shown in Figure 28.6 to establish a bulk velocity of 200cm/s. The trichlorosilane is reduced by the H2 gas to elemental silicon solid (Si), which is deposited as a thin film on a 15cm by 15cm wafer. Although the reaction produces HCl gas, it is significantly diluted by the H2 gas, and so the H2 gas can also be considered as the carrier gas. The flow over the silicon wafer is approximated as flow over a flat plate. The reaction process is maintained at 1.0atm and 1200K. The reactor contains elaborate safety systems to handle the toxic and flammable gases. At 1200K, the Figure 28.6 Chemical vapor deposition of solid silicon onto a wafer surface by a flowing gas stream mixture of trichlorosilane and H2, Example 3 . surface reaction constant for the first-order decomposition reaction with respect to SiHCl3 vapor concentration is ks=0.83cm/s, with the surface reaction rate law defined as RA,s=kscAs, based on the studies of Stein. 3
28.6 A horizontal chemical vapor deposition (CVD) reactor similar to the configuration shown in Example 3, Figure 28.6 will be used for growth of gallium arsenide (GaAs) thin films. In this process, arsine vapor, trimethylgallium vapor, and H2 gas are fed into the reactor. Inside the reactor, the silicon wafer rests on a heated plate called a susceptor. The reactant gases flow parallel to the surface of the wafer and deposit a GaAs thin film according to the simplified CVD reactions 2AsH3(g)2As(s)+3H2(g)2Ga(CH3)3(g)+3H2(g)2Ga(s)+6CH4(g) If the reactant gas is considerably diluted in H2 gas, then the mass transfer of each species in the H2 carrier gas can be treated separately. These surface reactions are considered to be very rapid, and so the mass transfer of the gaseous reactants to the surface of the wafer limits the rate of GaAs thin-film formation. In the present process, a 15cm15cm square silicon wafer is positioned at the leading edge of the susceptor plate. The process temperature is 800K, and the total system pressure 101.3kPa (1.0 atm). The feed gas delivered to the reactor results in a bulk linear velocity of 100cm/s. The composition of arsine and trimethylgallium in the feed gas are both 0.10 mole\%, which is very dilute. You may assume that the amount of arsine and trimethylgallium delivered with the feed gas is much higher than the amount of arsine and trimethylgallium consumed by the reactions, so that the concentration of these reactants in the bulk gas phase is essentially constant down the length of the reactor. You may also assume that the surface-reaction rates are instantaneous relative to the rates of mass transfer, so that the gasphase concentrations of both arsine vapor and trimethylgallium vapor at the surface of the wafer are essentially zero. The binary gas-phase diffusion coefficient of trimethylgallium in H2 is 1.55 cm2/s at 800K and 1.0atm. a. What are the average mass-transfer rates for arsine and trimethylgallium over the whole wafer? b. Based on the ratio of the arsine and trimethylgallium masstransfer rates, what is the composition of the GaAs composite thin film-e.g., the molar composition of gallium (Ga) and arsenic (As) in the solid? How could the feed-gas composition be adjusted so that the molar ratio of Ga to As within the solid thin film is 1:1 ? A chemical vapor deposition (CVD) process is used to deposit pure silicon thin films on wafers for electronic device applications. One way to deposit pure silicon onto a surface is by the heterogeneous surface reaction: SiHCl3(g)+H2(g)Si(s)+3HCl(g) In the present process, 1.0 mole\% trichlorosilane ( SiHCl3 ) vapor (species A ) diluted in H2 gas (species B ) is fed to the CVD reactor shown in Figure 28.6 to establish a bulk velocity of 200cm/s. The trichlorosilane is reduced by the H2 gas to elemental silicon solid (Si), which is deposited as a thin film on a 15cm by 15cm wafer. Although the reaction produces HCl gas, it is significantly diluted by the H2 gas, and so the H2 gas can also be considered as the carrier gas. The flow over the silicon wafer is approximated as flow over a flat plate. The reaction process is maintained at 1.0atm and 1200K. The reactor contains elaborate safety systems to handle the toxic and flammable gases. At 1200K, the Figure 28.6 Chemical vapor deposition of solid silicon onto a wafer surface by a flowing gas stream mixture of trichlorosilane and H2, Example 3 . surface reaction constant for the first-order decomposition reaction with respect to SiHCl3 vapor concentration is ks=0.83cm/s, with the surface reaction rate law defined as RA,s=kscAs, based on the studies of Stein. 3 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


