Answered step by step
Verified Expert Solution
Question
1 Approved Answer
29.11 In a wastewater treatment process, we are concerned about the possible release of the aromatic hydrocarbon benzene to the atmosphere. In the present process,
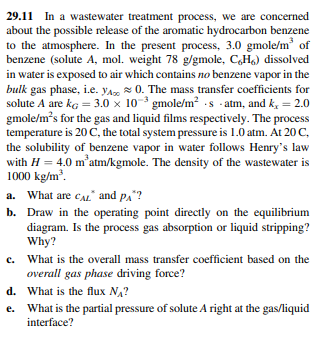 29.11 In a wastewater treatment process, we are concerned about the possible release of the aromatic hydrocarbon benzene to the atmosphere. In the present process, 3.0gmole/m3 of benzene (solute A, mol. weight 78g/gmole,C6H6 ) dissolved in water is exposed to air which contains no benzene vapor in the bulk gas phase, i.e. yA0. The mass transfer coefficients for solute A are kG=3.0103gmole/m2satm, and kx=2.0 gmole/ /m2s for the gas and liquid films respectively. The process temperature is 20C, the total system pressure is 1.0atm. At 20C, the solubility of benzene vapor in water follows Henry's law with H=4.0m3atm/kgmole. The density of the wastewater is 1000kg/m3. a. What are cSLx and pA ? b. Draw in the operating point directly on the equilibrium diagram. Is the process gas absorption or liquid stripping? Why? c. What is the overall mass transfer coefficient based on the overall gas phase driving force? d. What is the flux NA ? e. What is the partial pressure of solute A right at the gas/liquid interface
29.11 In a wastewater treatment process, we are concerned about the possible release of the aromatic hydrocarbon benzene to the atmosphere. In the present process, 3.0gmole/m3 of benzene (solute A, mol. weight 78g/gmole,C6H6 ) dissolved in water is exposed to air which contains no benzene vapor in the bulk gas phase, i.e. yA0. The mass transfer coefficients for solute A are kG=3.0103gmole/m2satm, and kx=2.0 gmole/ /m2s for the gas and liquid films respectively. The process temperature is 20C, the total system pressure is 1.0atm. At 20C, the solubility of benzene vapor in water follows Henry's law with H=4.0m3atm/kgmole. The density of the wastewater is 1000kg/m3. a. What are cSLx and pA ? b. Draw in the operating point directly on the equilibrium diagram. Is the process gas absorption or liquid stripping? Why? c. What is the overall mass transfer coefficient based on the overall gas phase driving force? d. What is the flux NA ? e. What is the partial pressure of solute A right at the gas/liquid interface Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


