Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hydrogen peroxide, H2O2, decomposes according to the equation above. This reaction is thermodynamically favorable at room temperature. (a) A particulate representation of the reactants
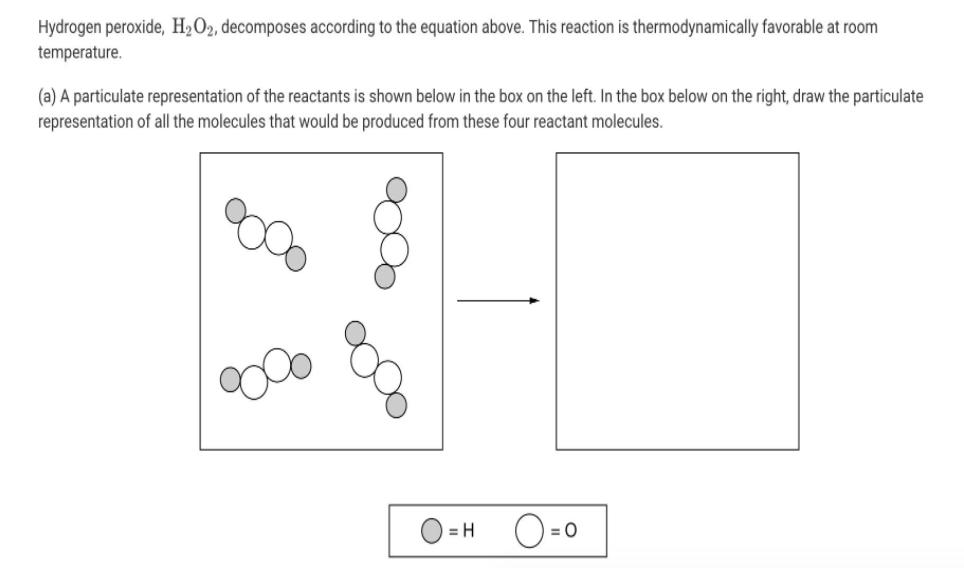
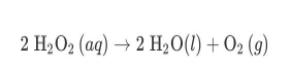
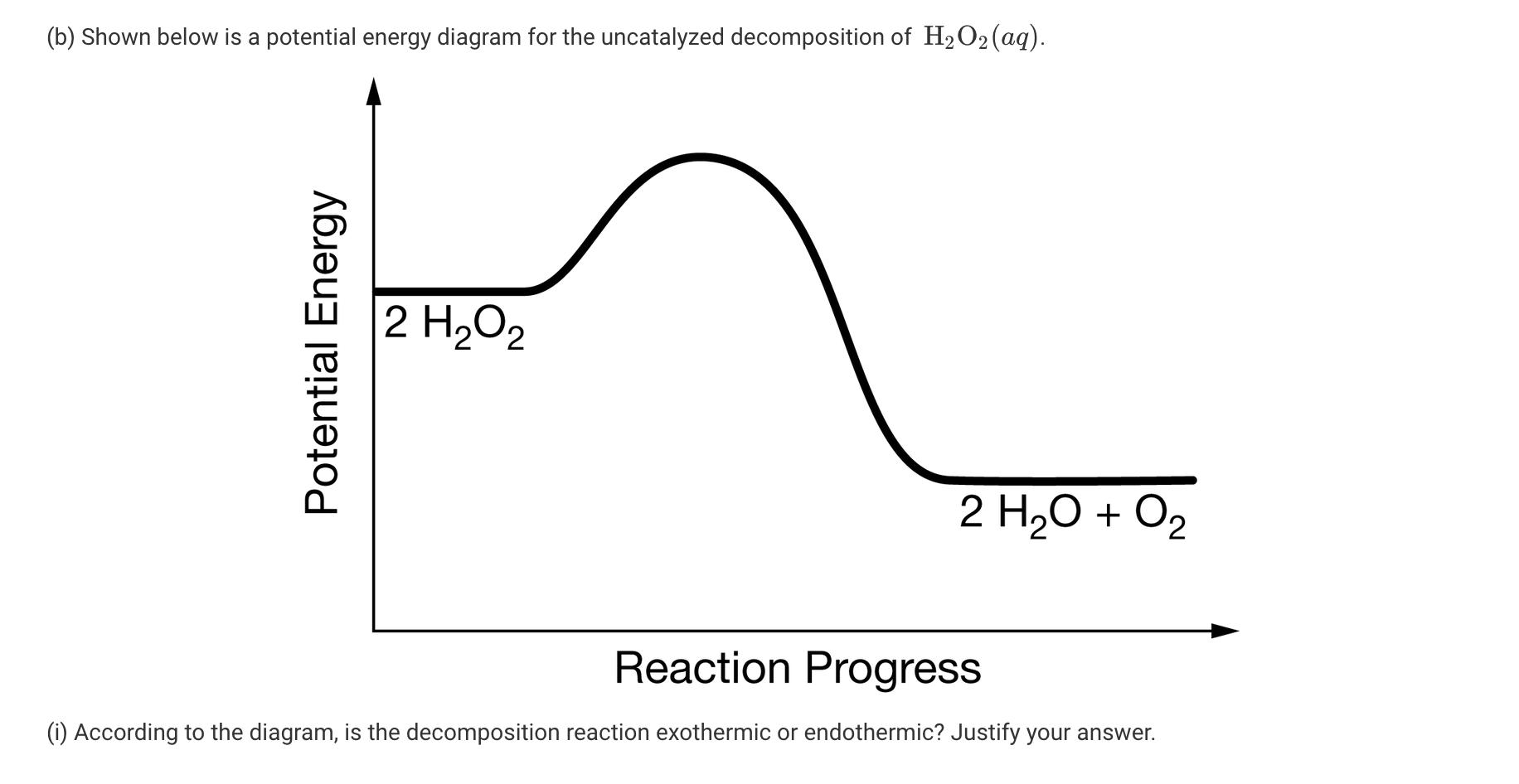
Hydrogen peroxide, H2O2, decomposes according to the equation above. This reaction is thermodynamically favorable at room temperature. (a) A particulate representation of the reactants is shown below in the box on the left. In the box below on the right, draw the particulate representation of all the molecules that would be produced from these four reactant molecules. O=0 = H 2 H2O2 (aq) 2 H20(1) + 02 (9) (b) Shown below is a potential energy diagram for the uncatalyzed decomposition of H2O2 (aq). 2 H2O2 2 H20 + O2 Reaction Progress (i) According to the diagram, is the decomposition reaction exothermic or endothermic? Justify your answer. Potential Energy (ii) Manganese dioxide, MnO2 (s), is an insoluble substance that acts as a catalyst for the decomposition reaction. On the diagram above, draw a curve to represent the reaction as it occurs in the presence of MnO2 (s). 1 Upload files (PDF, JPG, GIF, PNG, TXT, Word, Excel, Powerpoint, file formats supported) 0/2 File Limit
Step by Step Solution
★★★★★
3.31 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


