Question
Determine the dissociation constants for the acids. Express the answers in proper scientific notation where appropriate. Acid A: pKa Ka = = 4.0 Acid
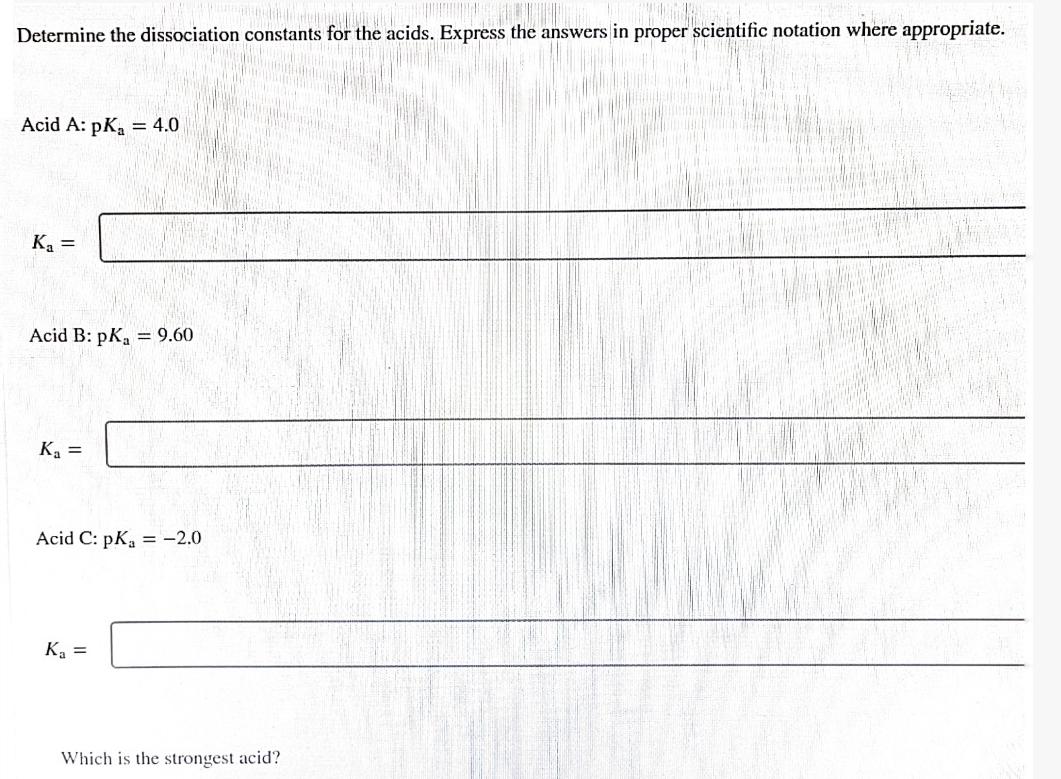
Determine the dissociation constants for the acids. Express the answers in proper scientific notation where appropriate. Acid A: pKa Ka = = 4.0 Acid B: pKa = 9.60 K = Acid C: pKa = -2.0 K = Which is the strongest acid?
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Engineering Economy
Authors: William G. Sullivan, Elin M. Wicks, C. Patrick Koelling
15th edition
132554909, 978-0132554909
Students also viewed these Finance questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



