Answered step by step
Verified Expert Solution
Question
1 Approved Answer
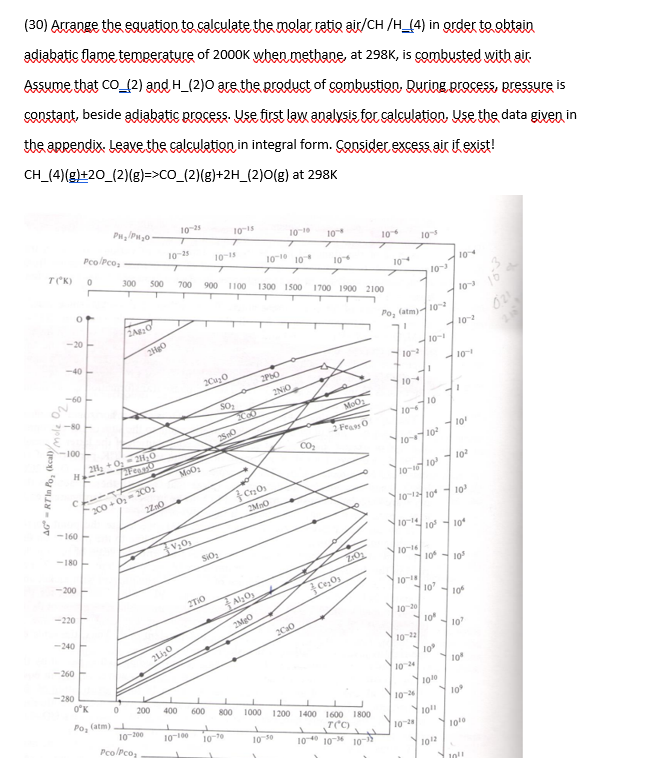
( 3 0 ) Arrange the equation to calculate the molar ratio air / CH / H 2 ( 4 ) in order to obtain
Arrange the equation to calculate the molar ratio airCH in order to obtain
adiabatic flame temperature of when methane, at is combusted with air.
Assume that and are the product of combustion, During process, pressure is
constant, beside adiabatic process. Use first law analysis for calculation, Use the data given in
the appendix. Leave the calculation in integral form. Consider excess air if exist!
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


