Answered step by step
Verified Expert Solution
Question
1 Approved Answer
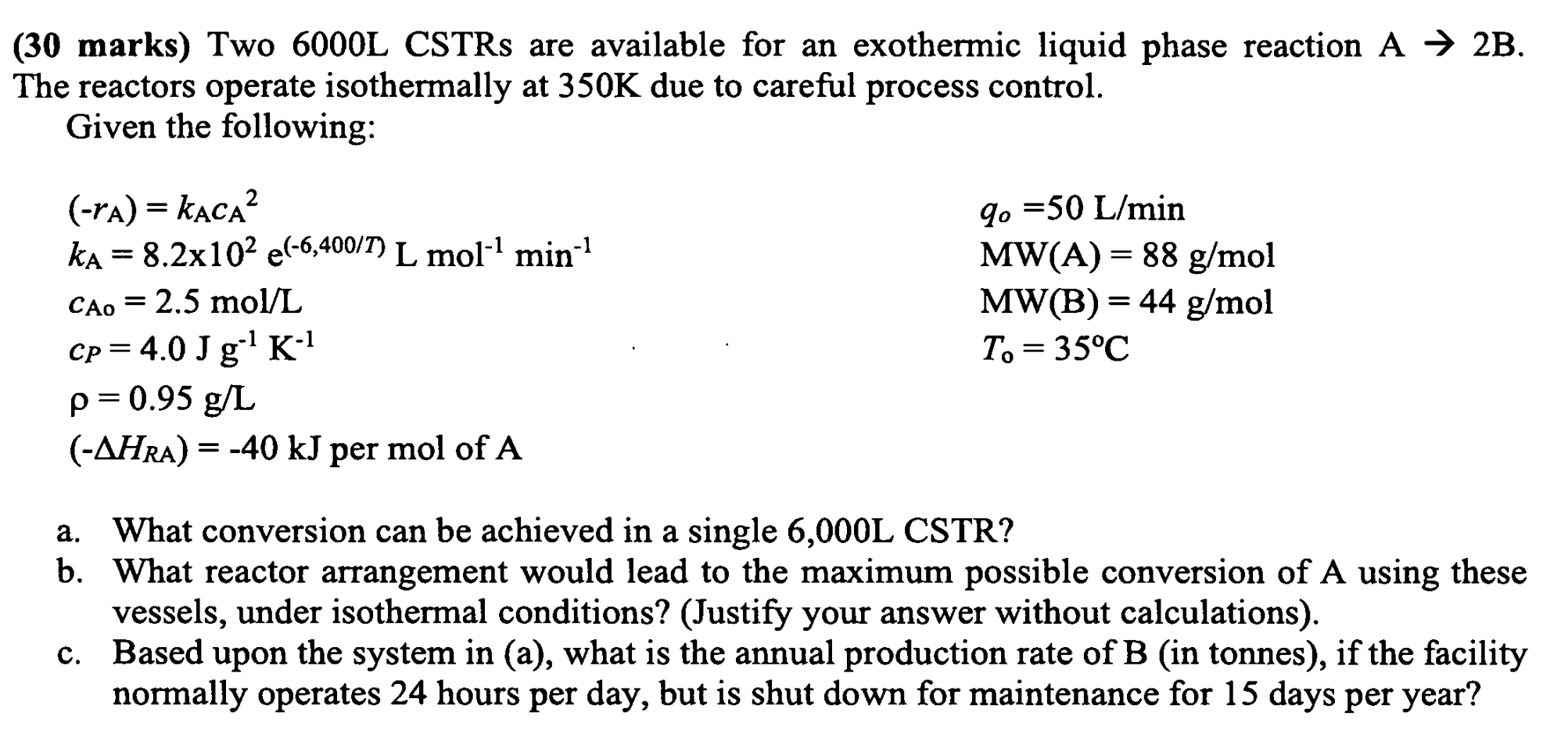
( 3 0 marks ) Two 6 0 0 0 L CSTRs are available for an exothermic liquid phase reaction A 2 B . The
marks Two L CSTRs are available for an exothermic liquid phase reaction
The reactors operate isothermally at due to careful process control.
Given the following:
per mol
a What conversion can be achieved in a single L CSTR
b What reactor arrangement would lead to the maximum possible conversion of A using these
vessels, under isothermal conditions? Justify your answer without calculations
c Based upon the system in a what is the annual production rate of B in tonnes if the facility
normally operates hours per day, but is shut down for maintenance for days per year?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


