Answered step by step
Verified Expert Solution
Question
1 Approved Answer
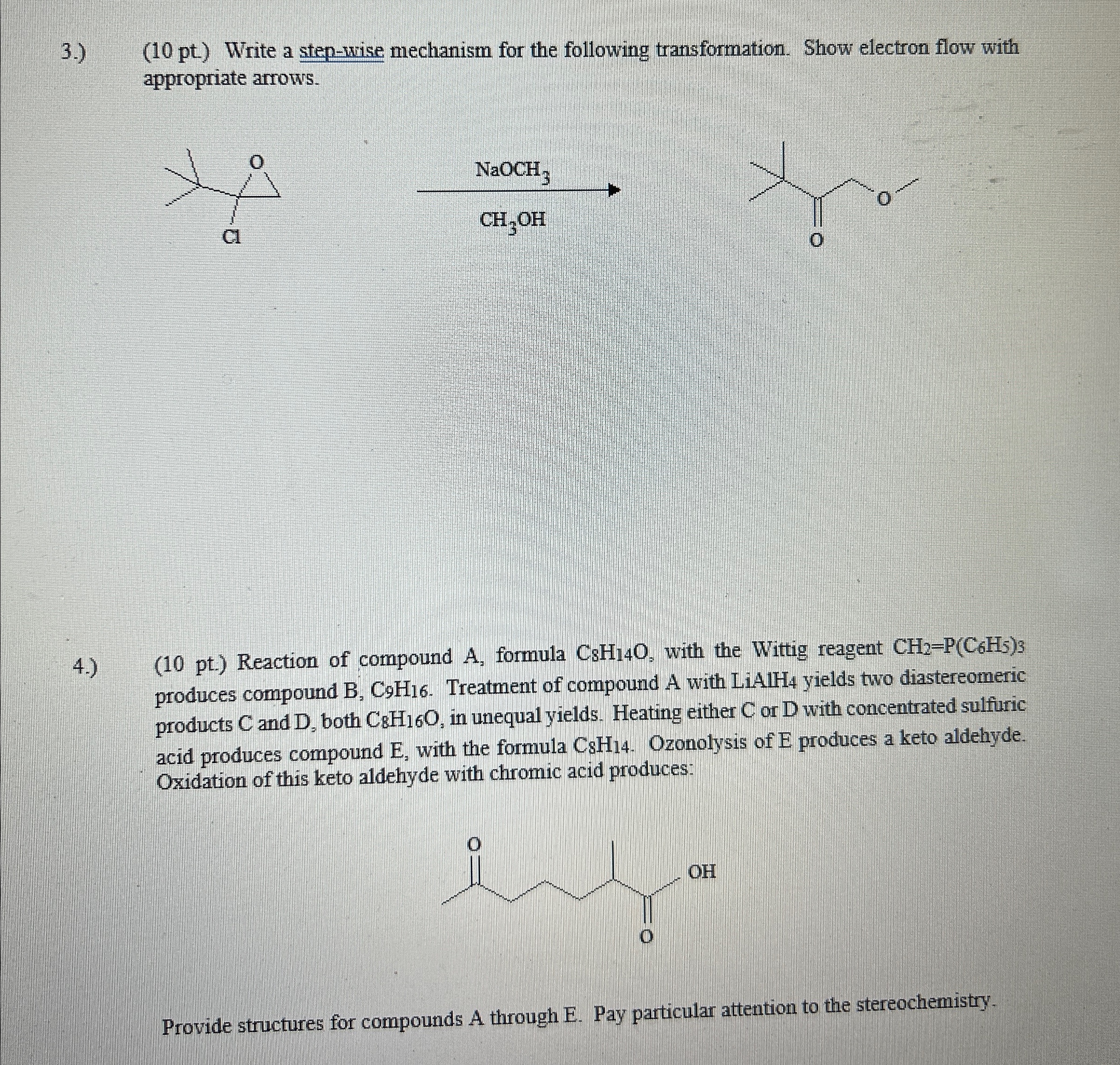
3 . ) ( 1 0 pt ) Write a step - wise mechanism for the following transformation. Show electron flow with appropriate arrows. ?
pt Write a stepwise mechanism for the following transformation. Show electron flow with appropriate arrows.
pt Reaction of compound formula with the Wittig reagent produces compound Treatment of compound A with yields two diastereomeric products and both in unequal yields. Heating either or with concentrated sulfuric acid produces compound with the formula Ozonolysis of produces a keto aldehyde. Oxidation of this keto aldehyde with chromic acid produces:
Provide structures for compounds A through E Pay particular attention to the stereochemistry.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


