Answered step by step
Verified Expert Solution
Question
1 Approved Answer
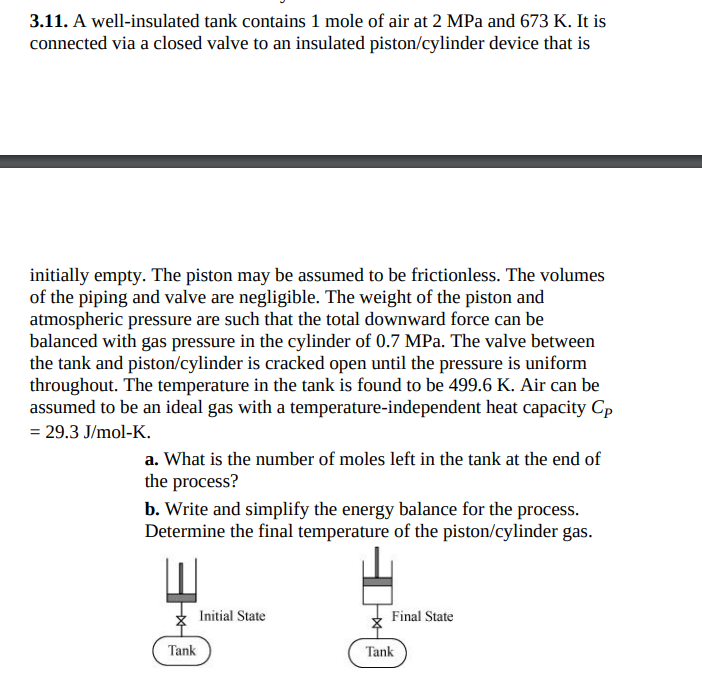
3 . 1 1 . A well - insulated tank contains 1 mole of air at 2 MPa and 6 7 3 K . It
A wellinsulated tank contains mole of air at MPa and It is
connected via a closed valve to an insulated pistoncylinder device that is
initially empty. The piston may be assumed to be frictionless. The volumes
of the piping and valve are negligible. The weight of the piston and
atmospheric pressure are such that the total downward force can be
balanced with gas pressure in the cylinder of MPa. The valve between
the tank and pistoncylinder is cracked open until the pressure is uniform
throughout. The temperature in the tank is found to be Air can be
assumed to be an ideal gas with a temperatureindependent heat capacity
a What is the number of moles left in the tank at the end of
the process?
b Write and simplify the energy balance for the process.
Determine the final temperature of the pistoncylinder gas.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


