Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. (10 points) Nitric oxide from car exhaust is a primary air pollutant. a) Calculate the equilibrium constant for the reaction N2(g) + O2(g) =
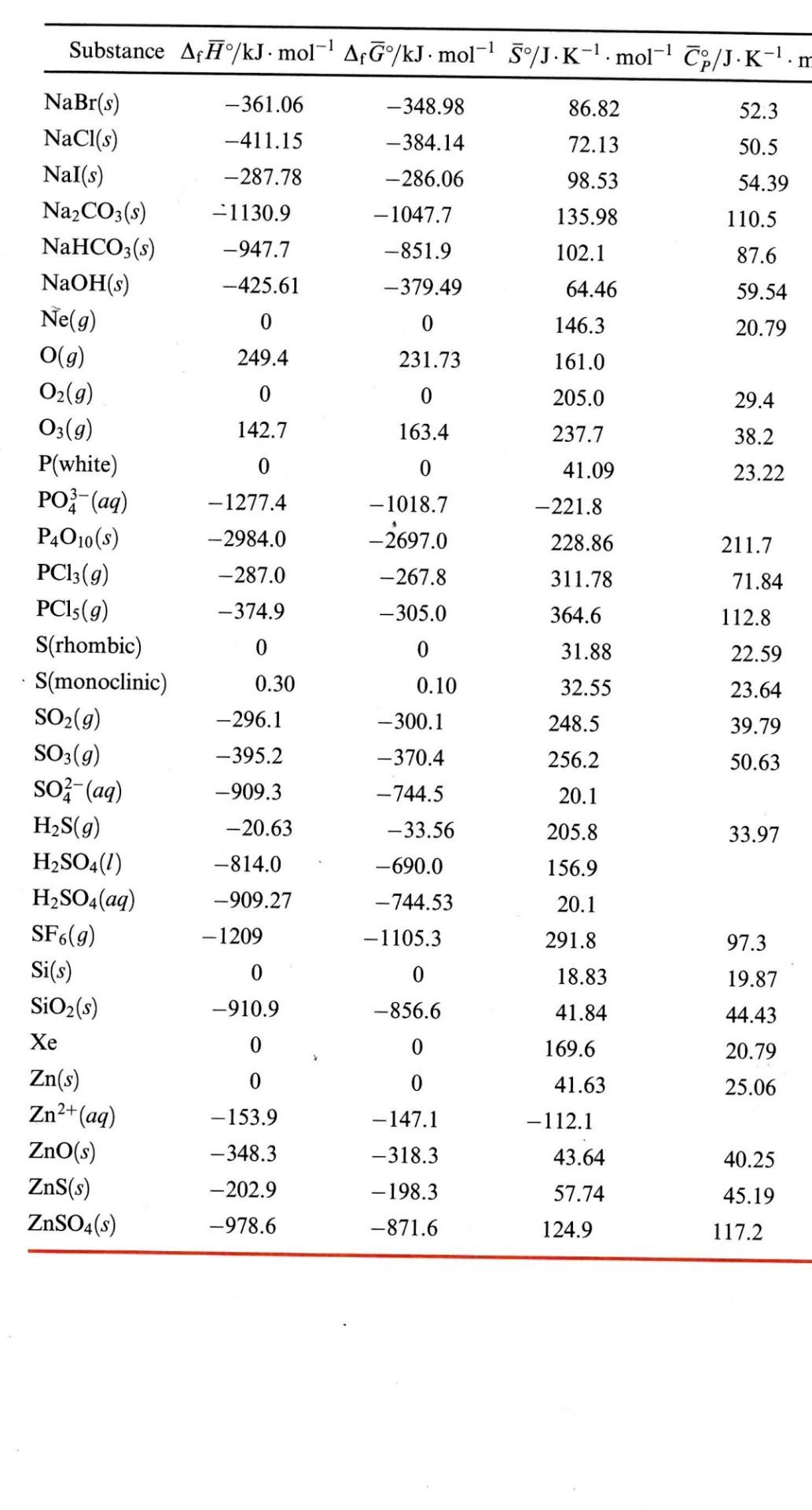
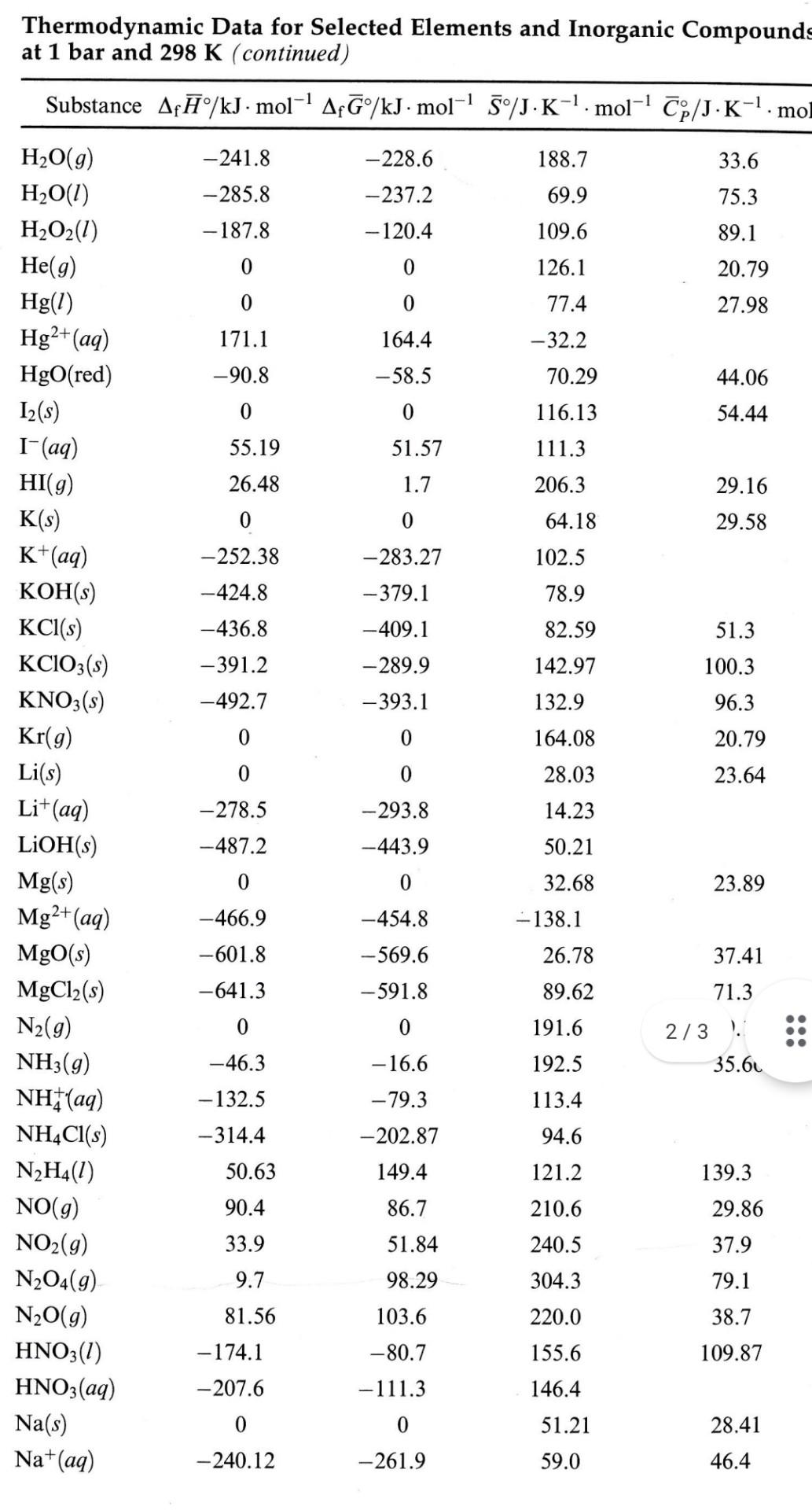
3. (10 points) Nitric oxide from car exhaust is a primary air pollutant. a) Calculate the equilibrium constant for the reaction N2(g) + O2(g) = 2 NO(g) at 25C using the data listed in the Thermodynamic Data on Moodle. b) At what temperature is the reaction, N2(8) + O2(g) = 2 NO(g), spontaneous? Substance Af/kJ. mol-' Af/kJ. mol-' 5/J.K-. mol-! p/J.K-'. 1 -1 -361.06 -348.98 86.82 52.3 -411.15 72.13 50.5 -287.78 98.53 54.39 -384.14 -286.06 -1047.7 -851.9 - 1130.9 135.98 110.5 -947.7 102.1 87.6 -425.61 - 379.49 64.46 146.3 59.54 20.79 0 0 249.4 231.73 161.0 0 0 205.0 29.4 142.7 163.4 237.7 38.2 0 41.09 23.22 -1277.4 -2984.0 -287.0 0 -1018.7 -2697.0 -221.8 228.86 211.7 -267.8 311.78 71.84 -374.9 -305.0 364.6 112.8 0 0 NaBr(s) NaCl(s) Nal(s) Na2CO3(s) NaHCO3(s) NaOH(s) Ne(g) O(g) O2(g) O3(g) P(white) PO3- (aq) P4010(s) PC13(g) PC15(9) S(rhombic) S(monoclinic) SO2(g) SO3(g) SO - (aq) H2S(g) H2SO4(1) H2SO4(aq) SF6(g) Si(s) SiO2(s) Xe Zn(s) Zn2+(aq) ZnO(s) ZnS(s) ZnSO4(s) 22.59 31.88 32.55 0.30 -296.1 0.10 - 300.1 -370.4 - 744.5 248.5 256.2 23.64 39.79 50.63 -395.2 -909.3 20.1 -33.56 33.97 205.8 156.9 -690.0 -20.63 -814.0 -909.27 - 1209 20.1 - 744.53 -1105.3 291.8 97.3 0 0 18.83 19.87 -910.9 -856.6 41.84 44.43 0 0 20.79 169.6 41.63 0 0 25.06 - 147.1 -112.1 -153.9 -348.3 40.25 -318.3 -198.3 -871.6 - 202.9 -978.6 43.64 57.74 124.9 45.19 117.2 Thermodynamic Data for Selected Elements and Inorganic Compounds at 1 bar and 298 K (continued) Substance Af/kJ. mol-' A?G/kJ. mol-' 5/J.K-1. mol-' Cp/J.K-. mol -228.6 188.7 33.6 -241.8 -285.8 -237.2 75.3 69.9 109.6 -187.8 - - 120.4 89.1 0 126.1 20.79 0 77.4 27.98 0 0 171.1 -90.8 2+ -32.2 164.4 - 58.5 44.06 70.29 116.13 0 0 54.44 55.19 51.57 111.3 26.48 1.7 206.3 29.16 0 0 64.18 29.58 -252.38 -424.8 -283.27 -379.1 102.5 78.9 -409.1 82.59 51.3 -436.8 -391.2 -289.9 142.97 100.3 -492.7 -393.1 132.9 96.3 0 0 164.08 20.79 0 0 23.64 H2O(g) H2O(1) H2O2(1) He(g) Hg(1) Hg2+ (aq) HgO(red) 12(s) I-(aq) HI(g) K(s) K+(aq) KOH(s) KCl(s) KCIO3(s) KNO3(s) Kr(g) Li(s) Li+(aq) LiOH(s) Mg(s) Mg2+ (aq) MgO(s) MgCl2(s) N2(g) NH3(g) NH(aq) NH4Cl(s) N2H4(1) NO(g) NO2(g) N204(9) N2O(g) HNO3(2) HNO3(aq) Na(s) Na+(aq) 28.03 14.23 -278.5 -293.8 -443.9 -487.2 50.21 0 0 32.68 23.89 -466.9 -454.8 -569.6 - 138.1 26.78 -601.8 37.41 -641.3 -591.8 71.3 89.62 191.6 0 0 2/3). 35.61 - 16.6 192.5 -46.3 -132.5 -79.3 113.4 -314.4 -202.87 149.4 94.6 121.2 50.63 139.3 90.4 86.7 210.6 29.86 37.9 33.9 51.84 240.5 9.7 98.29 304.3 79.1 81.56 103.6 220.0 38.7 -174.1 109.87 -80.7 -111.3 155.6 146.4 -207.6 0 0 51.21 28.41 - 240.12 -261.9 59.0 46.4
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


