Question
3. (30) A galvanic cell is composed of a silver half-cell and a and an iron half-cell. a) (15) If the silver half-cell is

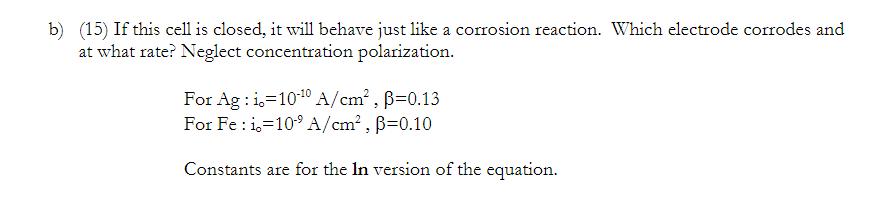
3. (30) A galvanic cell is composed of a silver half-cell and a and an iron half-cell. a) (15) If the silver half-cell is at 20C and the [Ag] is 2x10 mol/1 and the iron half-cell is at 35C and [Fe]2+ is 0.4 mol/1. What is the open cell potential? b) (15) If this cell is closed, it will behave just like a corrosion reaction. Which electrode corrodes and at what rate? Neglect concentration polarization. For Agi=10-10 A/cm, B=0.13 For Fei. 10 A/cm, B=0.10 Constants are for the In version of the equation.
Step by Step Solution
3.38 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
To solve this problem we need to follow these steps a Calculating the opencell potential The opencell potential of a galvanic cell is given by the Ner...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



