Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3) (35 pts) Consider the heterogeneous gas phase dehydrogenation of isopropanol to acetone using a solid Cu-SiO2 catalyst. (CH3)2CH(OH) I The reaction has the
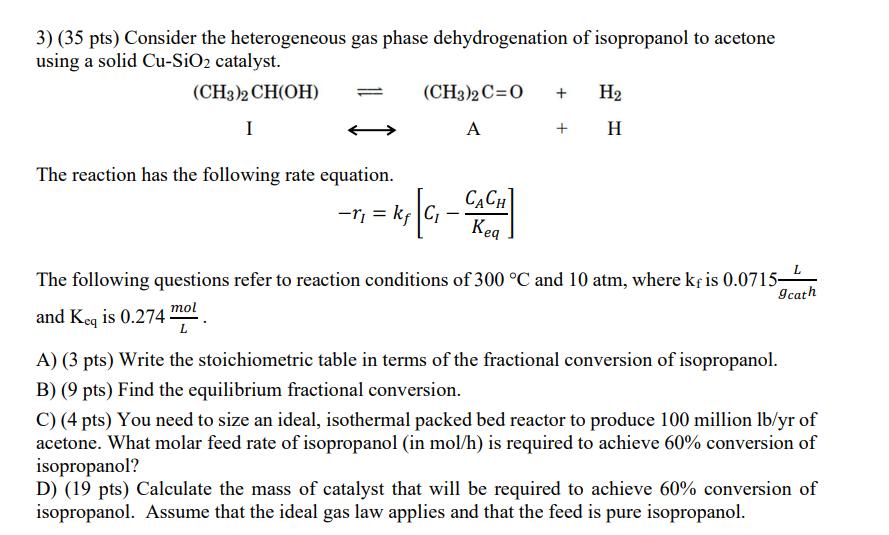
3) (35 pts) Consider the heterogeneous gas phase dehydrogenation of isopropanol to acetone using a solid Cu-SiO2 catalyst. (CH3)2CH(OH) I The reaction has the following rate equation. (CH3)2 C=O + H2 A + H - CACH Keq The following questions refer to reaction conditions of 300 C and 10 atm, where k is 0.0715 and Keq is 0.274 mol A) (3 pts) Write the stoichiometric table in terms of the fractional conversion of isopropanol. B) (9 pts) Find the equilibrium fractional conversion. L 9cath C) (4 pts) You need to size an ideal, isothermal packed bed reactor to produce 100 million lb/yr of acetone. What molar feed rate of isopropanol (in mol/h) is required to achieve 60% conversion of isopropanol? D) (19 pts) Calculate the mass of catalyst that will be required to achieve 60% conversion of isopropanol. Assume that the ideal gas law applies and that the feed is pure isopropanol.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


