Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. 4. Elevation in boiling point for 1.5 molal solution of glucose in water is 4 K. The depression in freezing point for 4.5
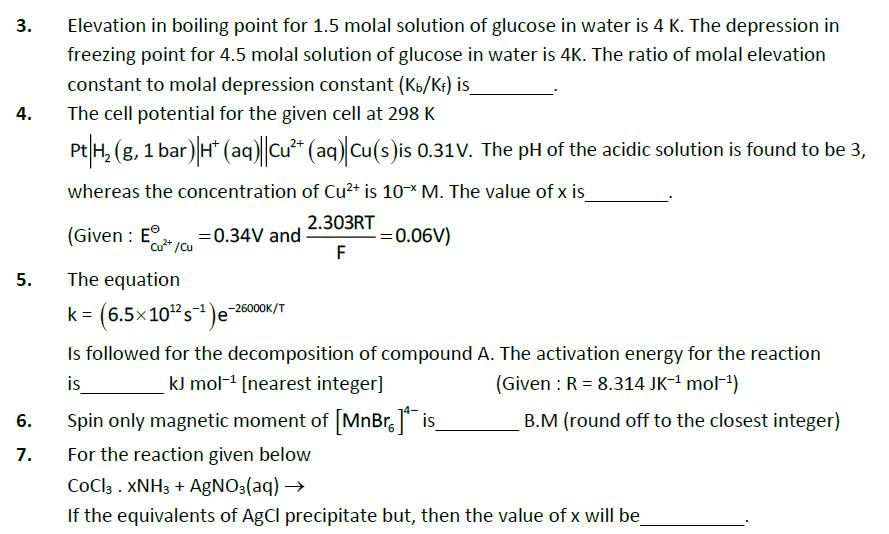
3. 4. Elevation in boiling point for 1.5 molal solution of glucose in water is 4 K. The depression in freezing point for 4.5 molal solution of glucose in water is 4K. The ratio of molal elevation constant to molal depression constant (Kb/K) is_ The cell potential for the given cell at 298 K Pt H2(g, 1 bar) H (aq) Cu+ (aq) Cu(s) is 0.31V. The pH of the acidic solution is found to be 3, whereas the concentration of Cu2+ is 10x M. The value of x is (Given: E+/cu 5. The equation =0.34V and 2.303RT F = 0.06V) k = (6.5x10 s)-26000K/T Is followed for the decomposition of compound A. The activation energy for the reaction kJ mol [nearest integer] (Given: R = 8.314 JK- mol-) is 6. 7. 19 Spin only magnetic moment of [MnBr.] is_ B.M (round off to the closest integer) For the reaction given below COCl3. XNH3 + AgNO3(aq) If the equivalents of AgCl precipitate but, then the value of x will be
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


