Answered step by step
Verified Expert Solution
Question
1 Approved Answer
( 3 5 p t ) Methane gas ( CH 4 ) enters a heat treatment furnace operating in steady state at 2 5 C
Methane gas CH enters a heat treatment furnace operating in steady state at and atm
pressure and reacts with theoretical air of the same temperature and pressure. Combustion
products come out at atm pressure and temperature. Determine;
a The balanced reaction equation for kmol of
b pt Air fuel ratio, at molar and mass base KmolairKmolMethane and kgairkgmethane
c Heat transfer amount for this reaction, in mol
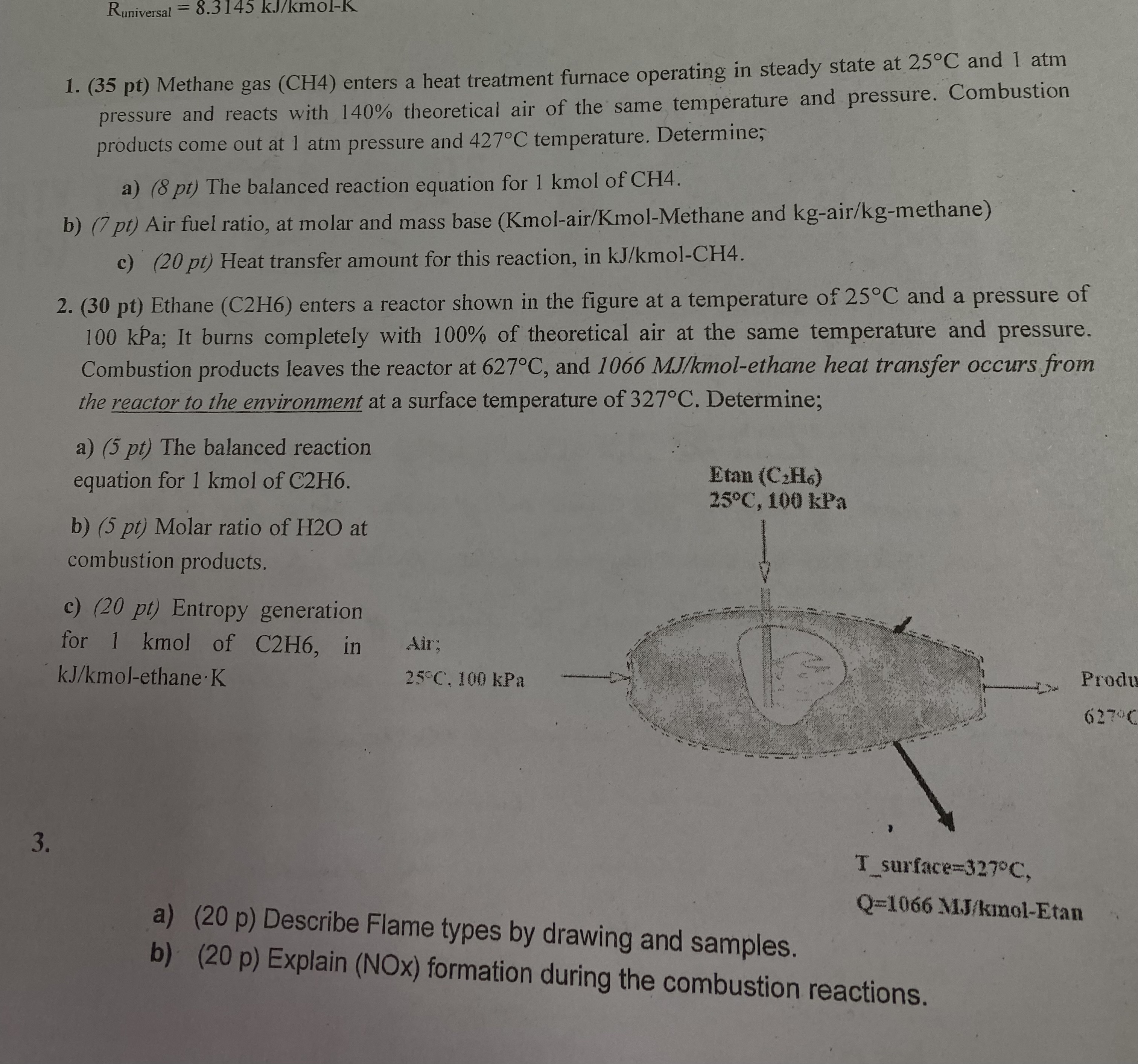
Ethane enters a reactor shown in the figure at a temperature of and a pressure of
kPa; It burns completely with of theoretical air at the same temperature and pressure.
Combustion products leaves the reactor at and molethane heat transfer occurs from
the reactor to the environment at a surface temperature of Determine;
a pt The balanced reaction
equation for kmol of
b pt Molar ratio of at
combustion products.
c Entropy generation
for kmol of in
molethane
a p Describe Flame types by drawing and samples.
b Explain NOx formation during the combustion reactions.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


