Answered step by step
Verified Expert Solution
Question
1 Approved Answer
( 3 5 points ) The irreversible elementary gas phase reaction 2 A B + C is currently carried out in a packed bed reactor
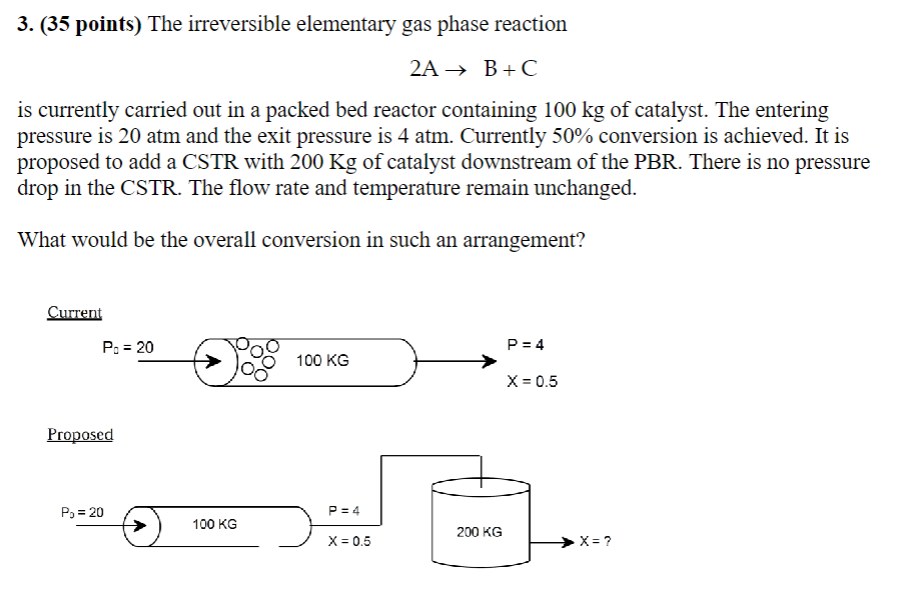
points The irreversible elementary gas phase reaction
is currently carried out in a packed bed reactor containing of catalyst. The entering
pressure is atm and the exit pressure is atm. Currently conversion is achieved. It is
proposed to add a CSTR with of catalyst downstream of the PBR There is no pressure
drop in the CSTR The flow rate and temperature remain unchanged.
What would be the overall conversion in such an arrangement?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


