Answered step by step
Verified Expert Solution
Question
1 Approved Answer
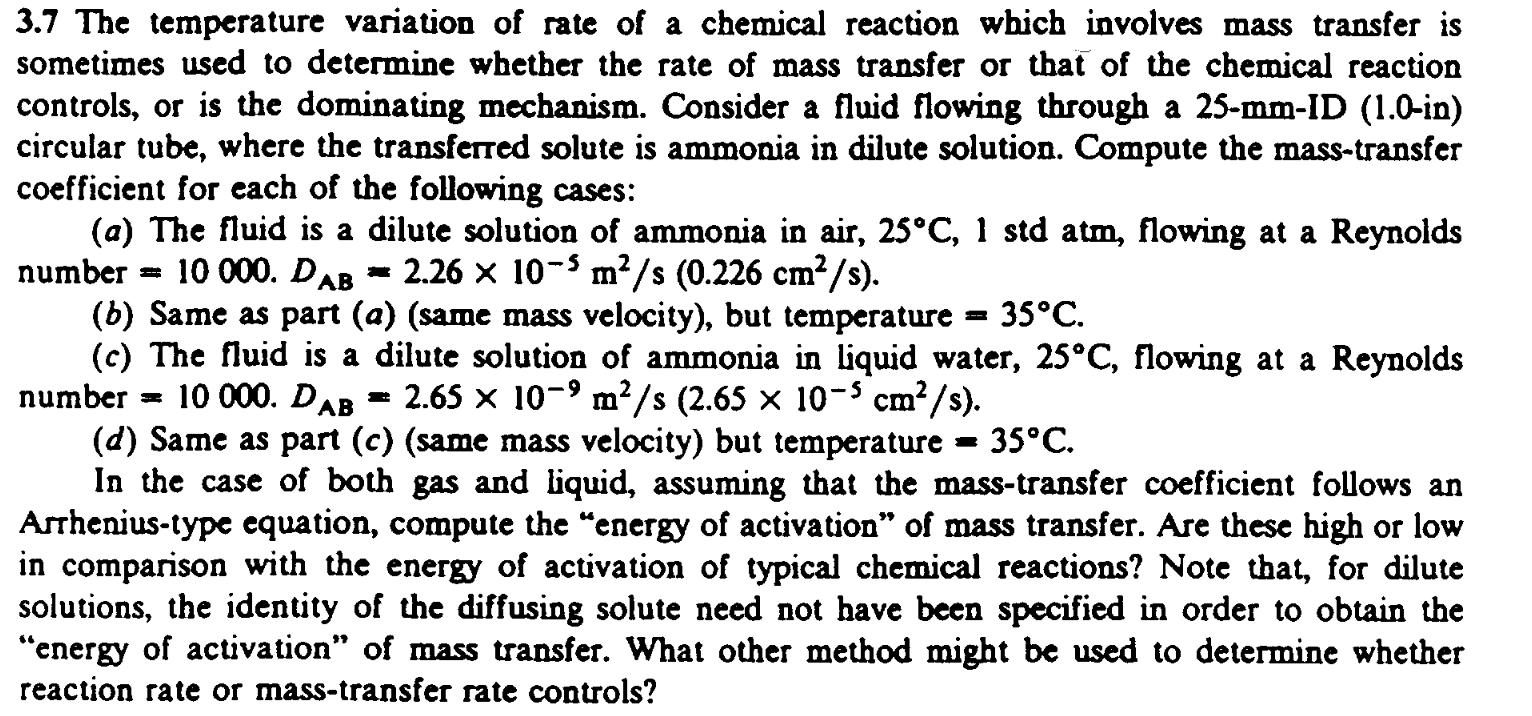
3 . 7 The temperature variation of rate of a chemical reaction which involves mass transfer is sometimes used to determine whether the rate of
The temperature variation of rate of a chemical reaction which involves mass transfer is
sometimes used to determine whether the rate of mass transfer or that of the chemical reaction
controls, or is the dominating mechanism. Consider a fluid flowing through a ID in
circular tube, where the transferred solute is ammonia in dilute solution. Compute the masstransfer
coefficient for each of the following cases:
a The fluid is a dilute solution of ammonia in air, std atm, flowing at a Reynolds
number
b Same as part asame mass velocity but temperature
c The fluid is a dilute solution of ammonia in liquid water, flowing at a Reynolds
number
d Same as part csame mass velocity but temperature
In the case of both gas and liquid, assuming that the masstransfer coefficient follows an
Arrheniustype equation, compute the "energy of activation" of mass transfer. Are these high or low
in comparison with the energy of activation of typical chemical reactions? Note that, for dilute
solutions, the identity of the diffusing solute need not have been specified in order to obtain the
"energy of activation" of mass transfer. What other method might be used to determine whether
reaction rate or masstransfer rate controls?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


