Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. A hypothetical element , transforms to phase from phase at 55K and 1atm. Consider and to be pure crystalline phases. Find the following: a.
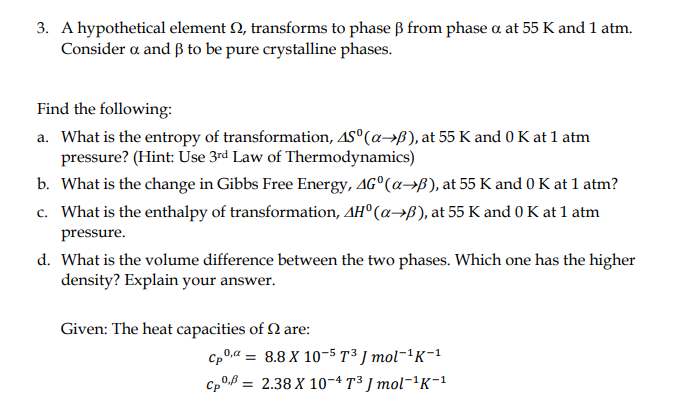 3. A hypothetical element , transforms to phase from phase at 55K and 1atm. Consider and to be pure crystalline phases. Find the following: a. What is the entropy of transformation, S0(), at 55K and 0K at 1atm pressure? (Hint: Use 3rd Law of Thermodynamics) b. What is the change in Gibbs Free Energy, G0(), at 55K and 0K at 1atm ? c. What is the enthalpy of transformation, H0(), at 55K and 0K at 1atm pressure. d. What is the volume difference between the two phases. Which one has the higher density? Explain your answer. Given: The heat capacities of are: cP0,=8.8105T3Jmol1K1cP0,=2.38104T3Jmol1K1 3. A hypothetical element , transforms to phase from phase at 55K and 1atm. Consider and to be pure crystalline phases. Find the following: a. What is the entropy of transformation, S0(), at 55K and 0K at 1atm pressure? (Hint: Use 3rd Law of Thermodynamics) b. What is the change in Gibbs Free Energy, G0(), at 55K and 0K at 1atm ? c. What is the enthalpy of transformation, H0(), at 55K and 0K at 1atm pressure. d. What is the volume difference between the two phases. Which one has the higher density? Explain your answer. Given: The heat capacities of are: cP0,=8.8105T3Jmol1K1cP0,=2.38104T3Jmol1K1
3. A hypothetical element , transforms to phase from phase at 55K and 1atm. Consider and to be pure crystalline phases. Find the following: a. What is the entropy of transformation, S0(), at 55K and 0K at 1atm pressure? (Hint: Use 3rd Law of Thermodynamics) b. What is the change in Gibbs Free Energy, G0(), at 55K and 0K at 1atm ? c. What is the enthalpy of transformation, H0(), at 55K and 0K at 1atm pressure. d. What is the volume difference between the two phases. Which one has the higher density? Explain your answer. Given: The heat capacities of are: cP0,=8.8105T3Jmol1K1cP0,=2.38104T3Jmol1K1 3. A hypothetical element , transforms to phase from phase at 55K and 1atm. Consider and to be pure crystalline phases. Find the following: a. What is the entropy of transformation, S0(), at 55K and 0K at 1atm pressure? (Hint: Use 3rd Law of Thermodynamics) b. What is the change in Gibbs Free Energy, G0(), at 55K and 0K at 1atm ? c. What is the enthalpy of transformation, H0(), at 55K and 0K at 1atm pressure. d. What is the volume difference between the two phases. Which one has the higher density? Explain your answer. Given: The heat capacities of are: cP0,=8.8105T3Jmol1K1cP0,=2.38104T3Jmol1K1 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


