Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. (a) The general formula of the structure factor for a cubic crystal can be written as: Fhkl fmcos (2(hxm + kym + lzm))
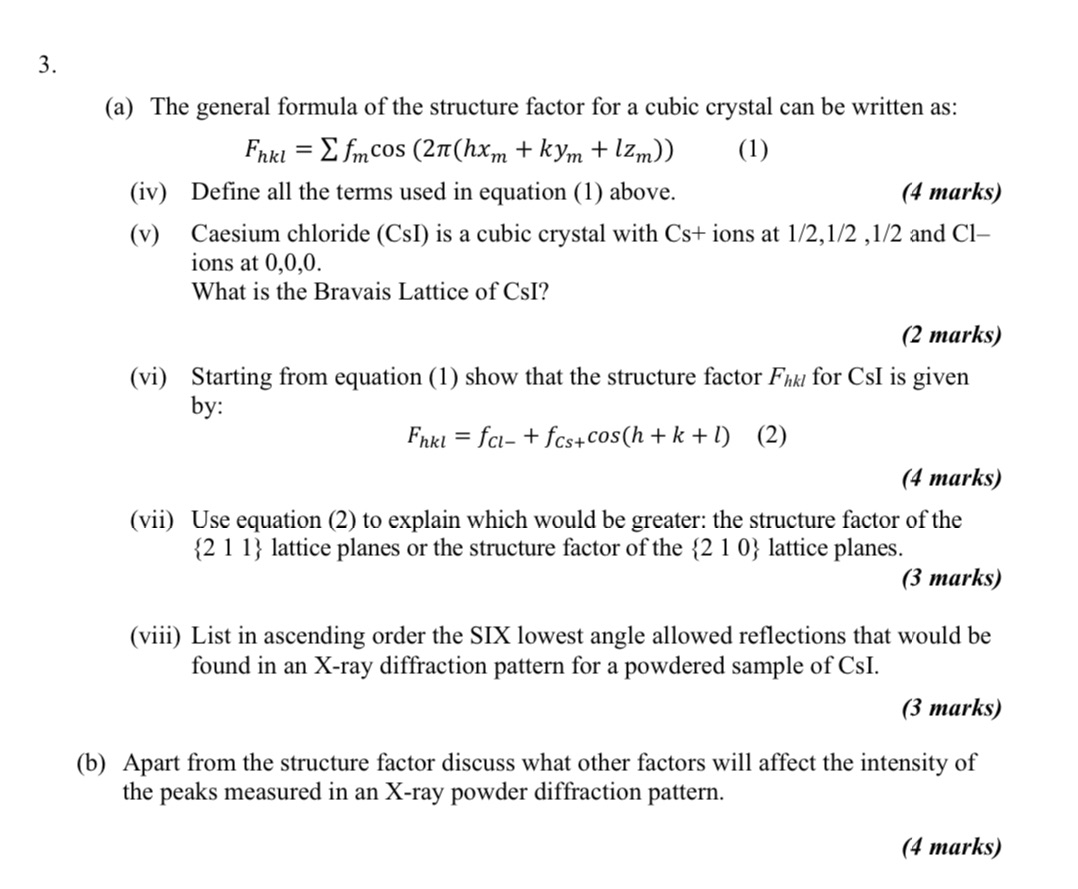
3. (a) The general formula of the structure factor for a cubic crystal can be written as: Fhkl fmcos (2(hxm + kym + lzm)) = (iv) Define all the terms used in equation (1) above. (v) (1) (4 marks) Caesium chloride (CSI) is a cubic crystal with Cs+ ions at 1/2,1/2,1/2 and Cl- ions at 0,0,0. What is the Bravais Lattice of CsI? (2 marks) (vi) Starting from equation (1) show that the structure factor Fhkl for CsI is given by: = Fhkl fcl-+fcs+ cos(h+k+1) (2) (4 marks) (vii) Use equation (2) to explain which would be greater: the structure factor of the {21 1} lattice planes or the structure factor of the {210} lattice planes. (3 marks) (viii) List in ascending order the SIX lowest angle allowed reflections that would be found in an X-ray diffraction pattern for a powdered sample of CSI. (3 marks) (b) Apart from the structure factor discuss what other factors will affect the intensity of the peaks measured in an X-ray powder diffraction pattern. (4 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


