Question
Reaction X-> Y is a single step reaction, with a deltaG (transition state) = 12000 J/(mol*K). At T=310K, the reaction rate is 0.10 M/s
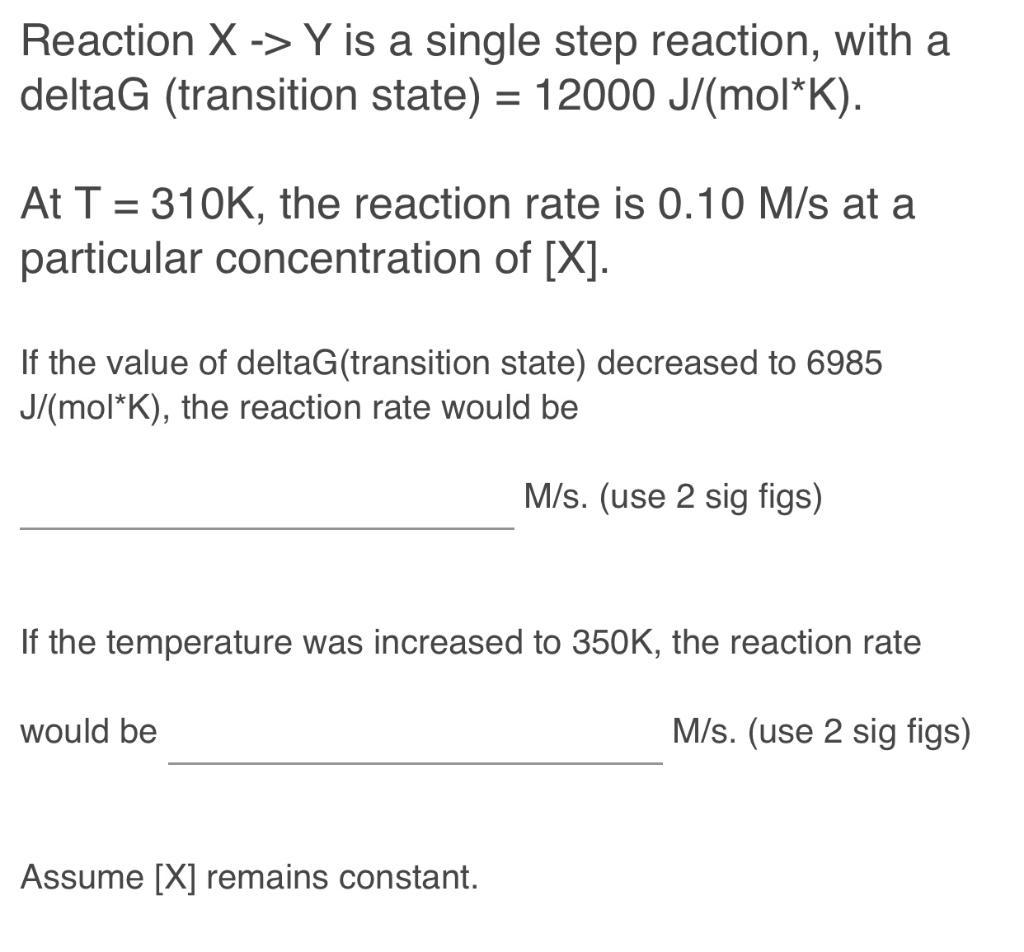
Reaction X-> Y is a single step reaction, with a deltaG (transition state) = 12000 J/(mol*K). At T=310K, the reaction rate is 0.10 M/s at a particular concentration of [X]. If the value of deltaG(transition state) decreased to 6985 J/(mol*K), the reaction rate would be M/s. (use 2 sig figs) If the temperature was increased to 350K, the reaction rate would be Assume [X] remains constant. M/s. (use 2 sig figs)
Step by Step Solution
3.43 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
The reaction rate constant k for a singlestep reaction can be calculated using the Arrhenius equatio...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
College Physics With An Integrated Approach To Forces And Kinematics
Authors: Alan Giambattista
5th Edition
126054771X, 978-1260547719
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



