Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. An ideal gas has a molar mass of 20 kg/kmol and a ratio of specific heats y = 1.20. It enters a rocket
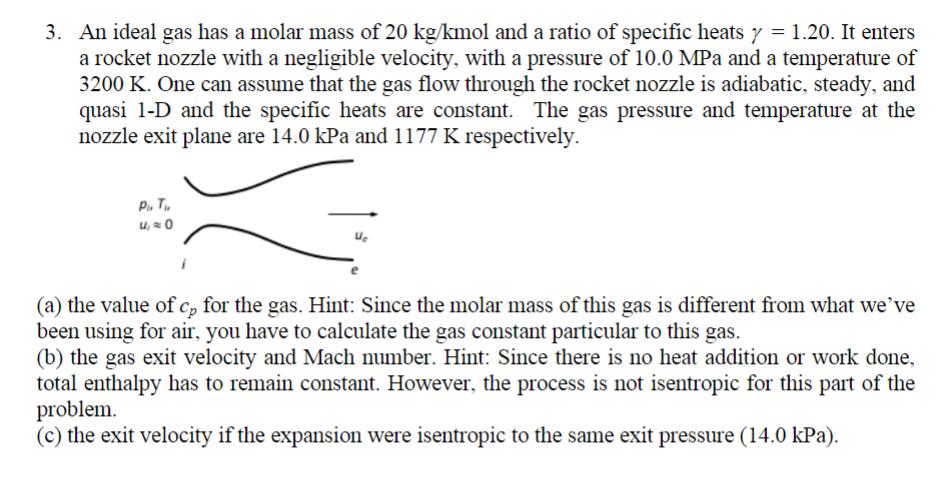
3. An ideal gas has a molar mass of 20 kg/kmol and a ratio of specific heats y = 1.20. It enters a rocket nozzle with a negligible velocity, with a pressure of 10.0 MPa and a temperature of 3200 K. One can assume that the gas flow through the rocket nozzle is adiabatic, steady, and quasi 1-D and the specific heats are constant. The gas pressure and temperature at the nozzle exit plane are 14.0 kPa and 1177 K respectively. P. T (a) the value of cp for the gas. Hint: Since the molar mass of this gas is different from what we've been using for air, you have to calculate the gas constant particular to this gas. (b) the gas exit velocity and Mach number. Hint: Since there is no heat addition or work done, total enthalpy has to remain constant. However, the process is not isentropic for this part of the problem. (c) the exit velocity if the expansion were isentropic to the same exit pressure (14.0 kPa).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


