Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3.) Calculate the concentrations of all molecular and ionic species and the pH in aqueous solutions that have the following formal compositions: a) 0.05

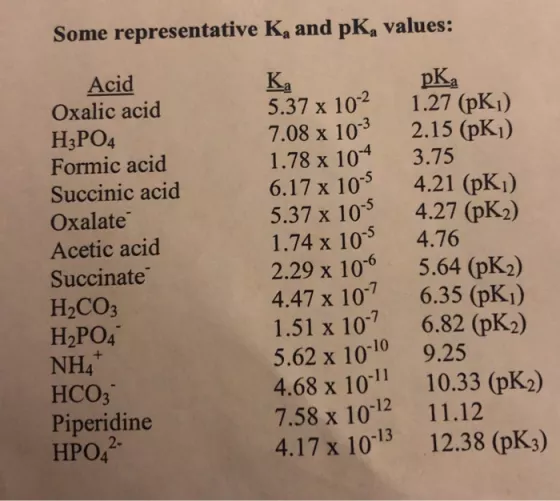
3.) Calculate the concentrations of all molecular and ionic species and the pH in aqueous solutions that have the following formal compositions: a) 0.05 M acetic acid + 0.1 M sodium acetate; b) 0.2 M boric acid + 0.05 M sodium borate (pK, of boric acid = 9.24); c) 0.5 M hydrochloric acid. Some representative K, and pK, values: Acid Oxalic acid Ka 5.37 x 102 7.08 x 103 1.78 x 104 6.17 x 105 5.37 x 105 1.74 x 105 2.29 x 106 4.47 x 107 1.51 x 107 5.62 x 1010 4.68 x 1011 7.58 x 1012 4.17 x 1013 pKa 1.27 (pK1) 2.15 (pK1) 3.75 H3PO4 Formic acid Succinic acid 4.21 (pK1) 4.27 (2) Oxalate Acetic acid 4.76 Succinate H2CO3 H2PO4 NH4 HCO; 5.64 (pK2) 6.35 (pK1) 6.82 (pK2) 9.25 10.33 (pK2) Piperidine HPO,2 11.12 12.38 (pK3)
Step by Step Solution
★★★★★
3.36 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1
Gi ven Data Some vepresen tati ve ka and pka values Acid ka pka Oralie acid ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


