Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3 different questions! Calculate the molarty of a solution made by diluting 10.9mL of 0.723MHCl solution to volurne of 406 mL. Round your answer to
3 different questions! 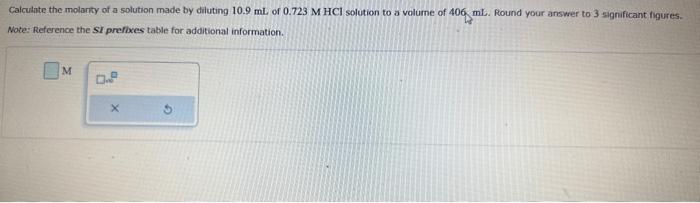
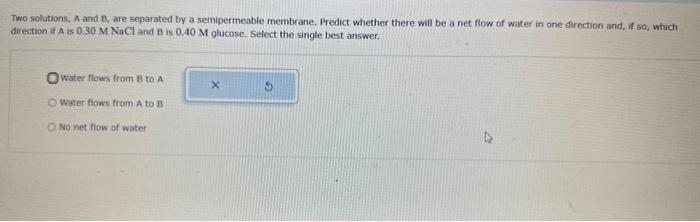
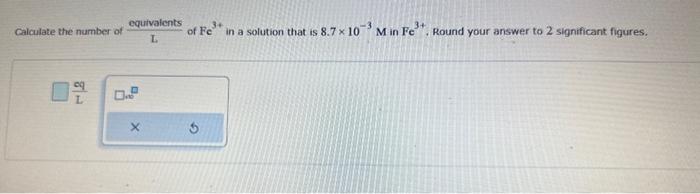
Calculate the molarty of a solution made by diluting 10.9mL of 0.723MHCl solution to volurne of 406 mL. Round your answer to 3 significant figures. Note: Reference the si prefioces table for additional information. Two solutions, A and B, are separated by a semipermeable membrane. Predict whether there will be a net flow of water in one direction and, if so, which direction if A is 0.30MNaCl and B is 0.40M glucose. Select the single best answer. Water flows from 8 to A Water flaws from A to B No net fiow of water Calculate the number of Lequivalents of Fe3+ in a solution that is 8.7103MinFe3+. Round your answer to 2 significant figures. Lc 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


