Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. John Dalton knew of the existence of at least three Oxides of Nitrogen. At the time, these were known as: Nitrous Gas, Nitrous
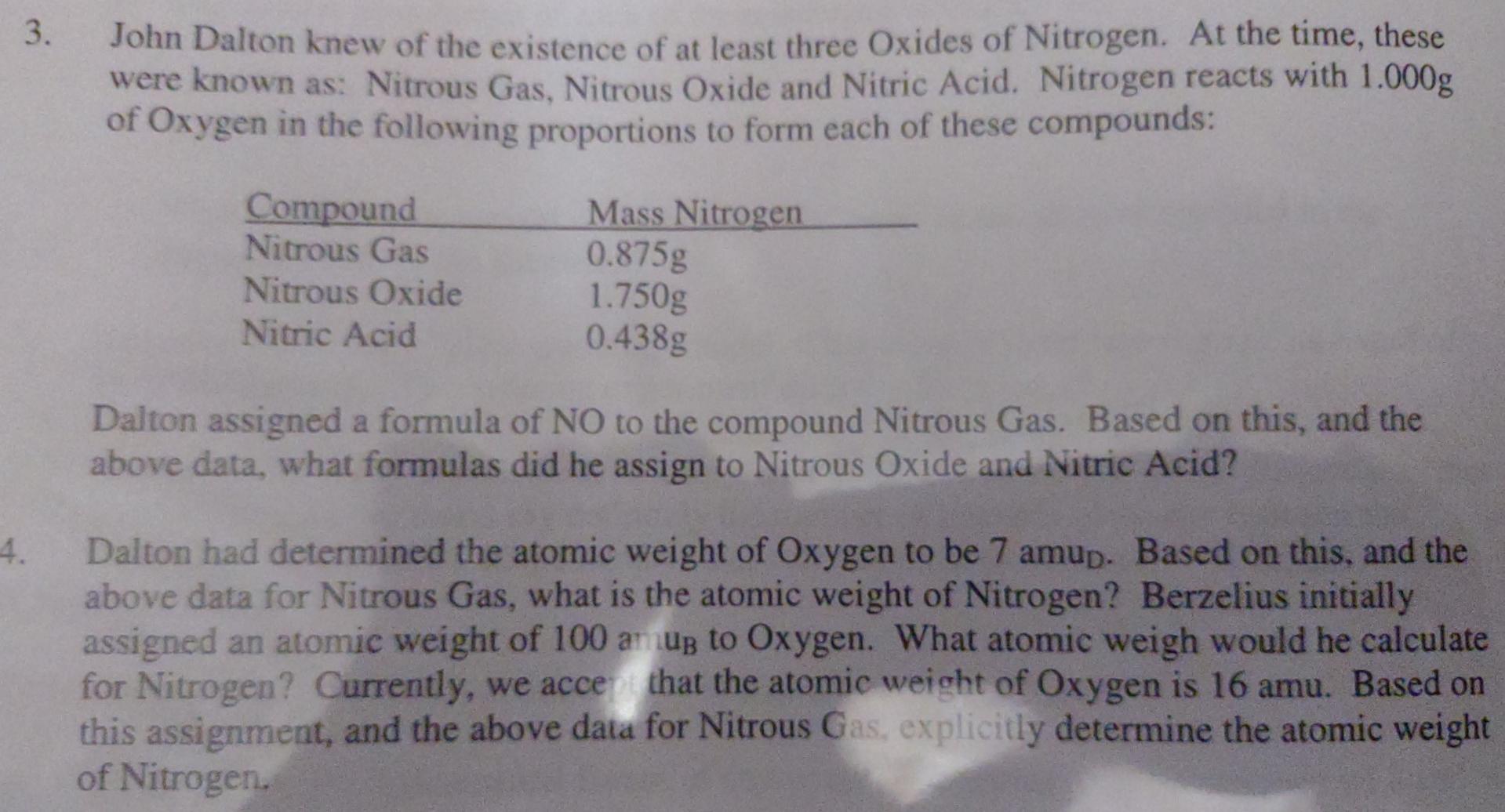
3. John Dalton knew of the existence of at least three Oxides of Nitrogen. At the time, these were known as: Nitrous Gas, Nitrous Oxide and Nitric Acid. Nitrogen reacts with 1.000g of Oxygen in the following proportions to form each of these compounds: Compound Nitrous Gas Mass Nitrogen 0.875g Nitrous Oxide Nitric Acid 1.750g 0.438g 4. Dalton assigned a formula of NO to the compound Nitrous Gas. Based on this, and the above data, what formulas did he assign to Nitrous Oxide and Nitric Acid? Dalton had determined the atomic weight of Oxygen to be 7 amup. Based on this, and the above data for Nitrous Gas, what is the atomic weight of Nitrogen? Berzelius initially assigned an atomic weight of 100 amup to Oxygen. What atomic weigh would he calculate for Nitrogen? Currently, we accept that the atomic weight of Oxygen is 16 amu. Based on this assignment, and the above data for Nitrous Gas, explicitly determine the atomic weight of Nitrogen.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
3 Based on Daltons assignment of NO to Nitrous Gas we can determine the formulas he would have assig...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
663e35bd6009a_959660.pdf
180 KBs PDF File
663e35bd6009a_959660.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


