Question
Titration of a 100.0 ml solution of certain weak acid and a strong base resulted in the below titration curve that asymptotically tends towards 12.0
Titration of a 100.0 ml solution of certain weak acid and a strong base resulted in the below titration curve that asymptotically tends towards 12.0 at infinite strong base volumes. Using the curve, estimate
a) The concentration of the strong base.
b) Ka of the weak acid.
c) The concentration of the weak acid solution.
d) Sketch, on the same plot, values of α0 and α1s. 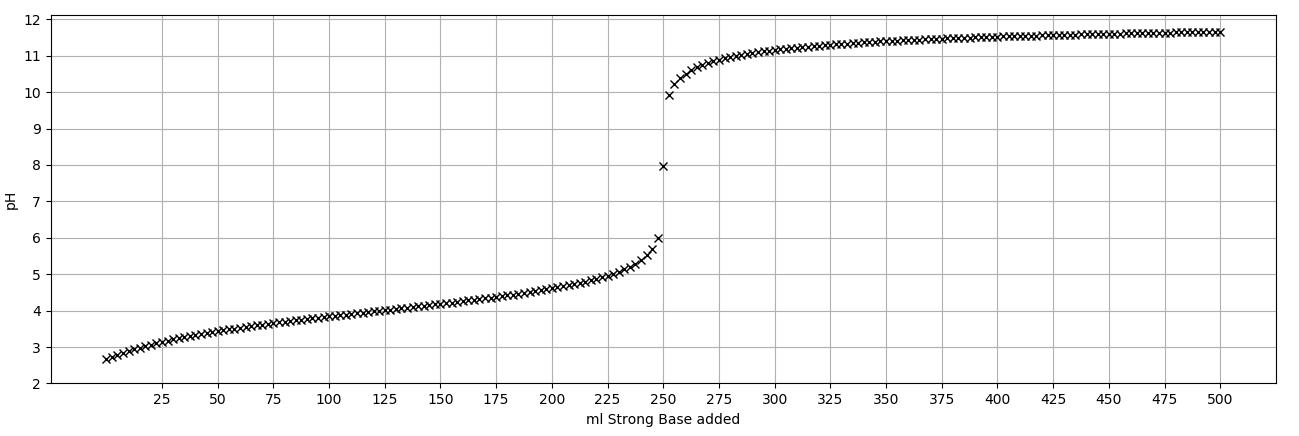
12 11 10 9 8 7 6 5 4 3 2 -5 25 50 75 100 125 150 175 200 225 250 275 ml Strong Base added 300 325 350 375 400 425 450 ****** 475 500
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solutions Step 1 a The concentration of ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Income Tax Fundamentals 2013
Authors: Gerald E. Whittenburg, Martha Altus Buller, Steven L Gill
31st Edition
1111972516, 978-1285586618, 1285586611, 978-1285613109, 978-1111972516
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



