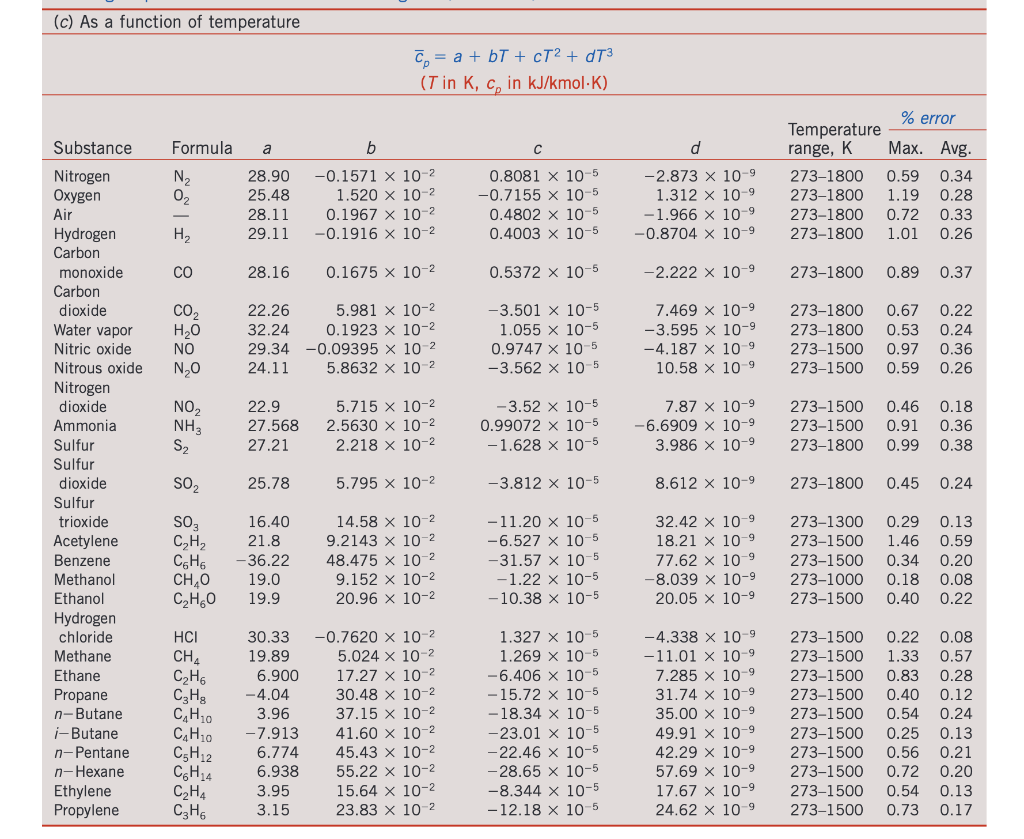
3. Propane enters a steady adiabatic compressor at 1 bar and 330 K and exits at 125 bar and 525 K. When propane behaves as an ideal gas, its specific heat is available as a function of temperature in Table A-2c (Cengel), Find the rate of entropy generation per unit mass flow rate, using the: (a) Peng-Robinson equation of state. When solving for Z1, use the largest root (which corresponds to the vapor phase, we'll discuss this later) (b) Generalized charts > (c) As a function of temperature C = a + bT + cT2 + dT3 (T in K, c, in kJ/kmol K) Formula a b d N2 OF 28.90 25.48 28.11 29.11 -0.1571 x 10-2 1.520 x 10-2 0.1967 x 10-2 -0.1916 x 10-2 0.8081 x 10-5 -0.7155 x 10-5 0.4802 x 10-5 0.4003 x 10-5 -2.873 x 10-9 1.312 x 10-9 -1.966 x 10-9 -0.8704 x 10-9 % error Temperature range, K Max. Avg. 273-1800 0.59 0.34 273-1800 1.19 0.28 273-1800 0.72 0.33 273-1800 1.01 0.26 H CO 28.16 0.1675 x 10-2 0.5372 x 10-5 -2.222 x 10-9 273-1800 0.89 0.37 CO2 HO NO NO 22.26 5.981 x 10-2 32.24 0.1923 x 10-2 29.34 -0.09395 x 10-2 24.11 5.8632 x 10-2 -3.501 x 10-5 1.055 x 10-5 0.9747 x 10-5 -3.562 x 10-5 7.469 x 10-9 -3.595 x 10-9 -4.187 X 10-9 10.58 x 10-9 273-1800 2731800 273-1500 273-1500 0.67 0.53 0.97 0.59 0.22 0.24 0.36 0.26 NOZ NH3 S2 22.9 27.568 27.21 5.715 x 10-2 2.5630 x 10-2 2.218 x 10-2 -3.52 x 10-5 0.99072 x 10-5 - 1.628 x 10-5 7.87 x 10-9 -6.6909 X 10-9 3.986 x 10-9 273-1500 273-1500 2731800 0.46 0.91 0.99 0.18 0.36 0.38 Substance Nitrogen Oxygen Air Hydrogen Carbon monoxide Carbon dioxide Water vapor Nitric oxide Nitrous oxide Nitrogen dioxide Ammonia Sulfur Sulfur dioxide Sulfur trioxide Acetylene Benzene Methanol Ethanol Hydrogen chloride Methane Ethane Propane n-Butane 1-Butane n-Pentane n-Hexane Ethylene Propylene SO 25.78 5.795 X 10-2 -3.812 x 10-5 8.612 x 10-9 273-1800 0.45 0.24 SO C,H, CEHO CH,0 C2H60 16.40 21.8 -36.22 19.0 19.9 14.58 x 10-2 9.2143 x 10-2 48.475 x 10-2 9.152 x 10-2 20.96 x 10-2 - 11.20 x 10-5 -6.527 x 10-5 -31.57 x 10-5 -1.22 x 10-5 -10.38 x 10-5 32.42 x 10-9 18.21 x 10-9 77.62 x 10-9 -8.039 x 10-9 20.05 x 10-9 273-1300 273-1500 273-1500 273-1000 273-1500 0.29 0.13 1.46 0.59 0.34 0.20 0.18 0.08 0.40 0.22 0.08 0.57 0.28 0.12 0.24 HCI CHA CH6 CzHg CH10 C4H10 CEH2 CH14 C2H4 C3H6 30.33 19.89 6.900 -4.04 3.96 -7.913 6.774 6.938 3.95 3.15 -0.7620 x 10-2 5.024 x 10-2 17.27 x 10-2 30.48 x 10-2 37.15 x 10-2 41.60 x 10-2 45.43 x 10-2 55.22 x 10-2 15.64 x 10-2 23.83 x 10-2 1.327 x 10-5 1.269 x 10-5 -6.406 x 10-5 - 15.72 x 10-5 - 18.34 x 10-5 -23.01 x 10-5 -22.46 x 10-5 -28.65 x 10-5 -8.344 x 10-5 - 12.18 x 10-5 -4.338 x 10-9 -11.01 x 10-9 7.285 x 10-9 31.74 x 10-9 35.00 x 10-9 49.91 x 10-9 42.29 x 10-9 57.69 x 10-9 17.67 X 10-9 24.62 x 10 9 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 0.22 1.33 0.83 0.40 0.54 0.25 0.56 0.72 0.54 0.73 0.13 0.21 0.20 0.13 0.17 3. Propane enters a steady adiabatic compressor at 1 bar and 330 K and exits at 125 bar and 525 K. When propane behaves as an ideal gas, its specific heat is available as a function of temperature in Table A-2c (Cengel), Find the rate of entropy generation per unit mass flow rate, using the: (a) Peng-Robinson equation of state. When solving for Z1, use the largest root (which corresponds to the vapor phase, we'll discuss this later) (b) Generalized charts > (c) As a function of temperature C = a + bT + cT2 + dT3 (T in K, c, in kJ/kmol K) Formula a b d N2 OF 28.90 25.48 28.11 29.11 -0.1571 x 10-2 1.520 x 10-2 0.1967 x 10-2 -0.1916 x 10-2 0.8081 x 10-5 -0.7155 x 10-5 0.4802 x 10-5 0.4003 x 10-5 -2.873 x 10-9 1.312 x 10-9 -1.966 x 10-9 -0.8704 x 10-9 % error Temperature range, K Max. Avg. 273-1800 0.59 0.34 273-1800 1.19 0.28 273-1800 0.72 0.33 273-1800 1.01 0.26 H CO 28.16 0.1675 x 10-2 0.5372 x 10-5 -2.222 x 10-9 273-1800 0.89 0.37 CO2 HO NO NO 22.26 5.981 x 10-2 32.24 0.1923 x 10-2 29.34 -0.09395 x 10-2 24.11 5.8632 x 10-2 -3.501 x 10-5 1.055 x 10-5 0.9747 x 10-5 -3.562 x 10-5 7.469 x 10-9 -3.595 x 10-9 -4.187 X 10-9 10.58 x 10-9 273-1800 2731800 273-1500 273-1500 0.67 0.53 0.97 0.59 0.22 0.24 0.36 0.26 NOZ NH3 S2 22.9 27.568 27.21 5.715 x 10-2 2.5630 x 10-2 2.218 x 10-2 -3.52 x 10-5 0.99072 x 10-5 - 1.628 x 10-5 7.87 x 10-9 -6.6909 X 10-9 3.986 x 10-9 273-1500 273-1500 2731800 0.46 0.91 0.99 0.18 0.36 0.38 Substance Nitrogen Oxygen Air Hydrogen Carbon monoxide Carbon dioxide Water vapor Nitric oxide Nitrous oxide Nitrogen dioxide Ammonia Sulfur Sulfur dioxide Sulfur trioxide Acetylene Benzene Methanol Ethanol Hydrogen chloride Methane Ethane Propane n-Butane 1-Butane n-Pentane n-Hexane Ethylene Propylene SO 25.78 5.795 X 10-2 -3.812 x 10-5 8.612 x 10-9 273-1800 0.45 0.24 SO C,H, CEHO CH,0 C2H60 16.40 21.8 -36.22 19.0 19.9 14.58 x 10-2 9.2143 x 10-2 48.475 x 10-2 9.152 x 10-2 20.96 x 10-2 - 11.20 x 10-5 -6.527 x 10-5 -31.57 x 10-5 -1.22 x 10-5 -10.38 x 10-5 32.42 x 10-9 18.21 x 10-9 77.62 x 10-9 -8.039 x 10-9 20.05 x 10-9 273-1300 273-1500 273-1500 273-1000 273-1500 0.29 0.13 1.46 0.59 0.34 0.20 0.18 0.08 0.40 0.22 0.08 0.57 0.28 0.12 0.24 HCI CHA CH6 CzHg CH10 C4H10 CEH2 CH14 C2H4 C3H6 30.33 19.89 6.900 -4.04 3.96 -7.913 6.774 6.938 3.95 3.15 -0.7620 x 10-2 5.024 x 10-2 17.27 x 10-2 30.48 x 10-2 37.15 x 10-2 41.60 x 10-2 45.43 x 10-2 55.22 x 10-2 15.64 x 10-2 23.83 x 10-2 1.327 x 10-5 1.269 x 10-5 -6.406 x 10-5 - 15.72 x 10-5 - 18.34 x 10-5 -23.01 x 10-5 -22.46 x 10-5 -28.65 x 10-5 -8.344 x 10-5 - 12.18 x 10-5 -4.338 x 10-9 -11.01 x 10-9 7.285 x 10-9 31.74 x 10-9 35.00 x 10-9 49.91 x 10-9 42.29 x 10-9 57.69 x 10-9 17.67 X 10-9 24.62 x 10 9 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 273-1500 0.22 1.33 0.83 0.40 0.54 0.25 0.56 0.72 0.54 0.73 0.13 0.21 0.20 0.13 0.17








