Question
Deuterium is an isotope of hydrogen, sometimes called heavy hydrogen. Hydrogen (H) has 1 electron, 1 proton, and 0 neutrons when neutral. Deuterium (D)
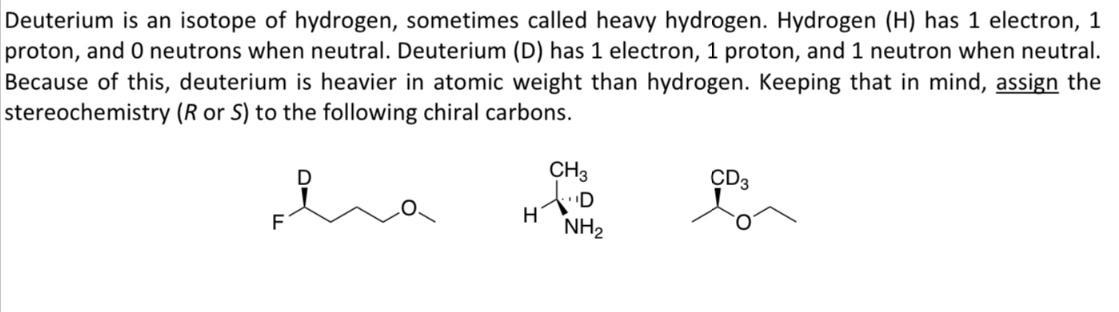
Deuterium is an isotope of hydrogen, sometimes called heavy hydrogen. Hydrogen (H) has 1 electron, 1 proton, and 0 neutrons when neutral. Deuterium (D) has 1 electron, 1 proton, and 1 neutron when neutral. Because of this, deuterium is heavier in atomic weight than hydrogen. Keeping that in mind, assign the stereochemistry (R or S) to the following chiral carbons. F H CH3 D CD3 NH2
Step by Step Solution
3.54 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Derivatives Markets
Authors: Robert McDonald
3rd Edition
978-9332536746, 9789332536746
Students also viewed these Finance questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



