Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. Suppose you burn 0.211 g of C(s) in an excess of O2(g) in a constant-V calorimeter to give CO2(g). C(s) + O2(g) = CO2(g)
3. Suppose you burn 0.211 g of C(s) in an excess of O2(g) in a constant-V calorimeter to give CO2(g).
Suppose you burn 0.211g of C(s) in an excess of O2(g) in a constant-V calorimeter to give CO2(g). C(s)+O2(g)CO2(g) The T of the calorimeter, which contained 871g of water, increased from 20.00 to 25.09C. The heat capacity of the bomb is 890 J/K. Calculate E per mole of carbon (enter in kJ/mol).4.184J/gC for H2O. Enter with correct sign and to 1 decimal place Question 4 2 pts Calculate the H for the following reaction from data given. A+3B 6C+2D C(s) + O2(g) = CO2(g)
The T of the calorimeter, which contained 871 g of water, increased from 20 to 25.09 degrees C. The heat capacity of the bomb is 890 J/K. Calculate E (delta E) per mole of carbon (enfer in kJ/mol). 4.184 J/g C for H2O.
4. Calculate the H (delta H) for the following reaction from data given.
A + 3B = 6C + 2D *see graph
Please answer both questions!! 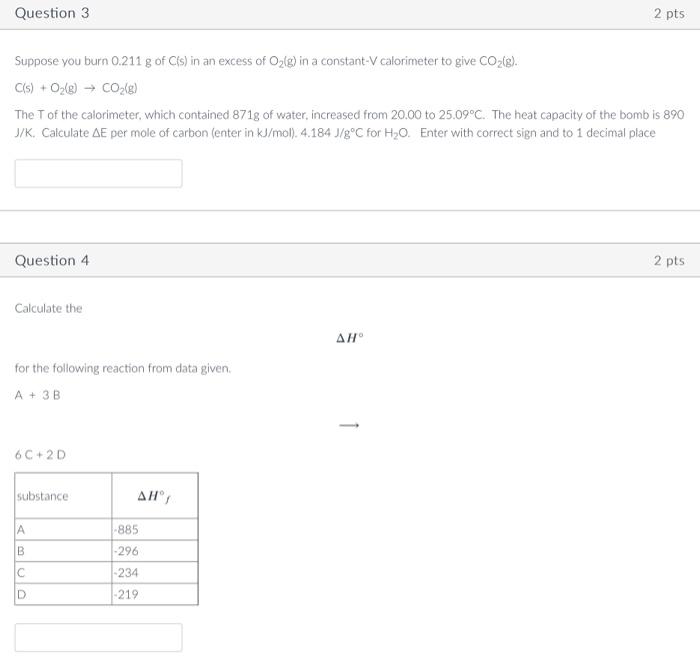

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


