Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. The sulfate ion concentration in natural water can be determined by measuring the turbidity that results when an excess of BaCl2 is added to
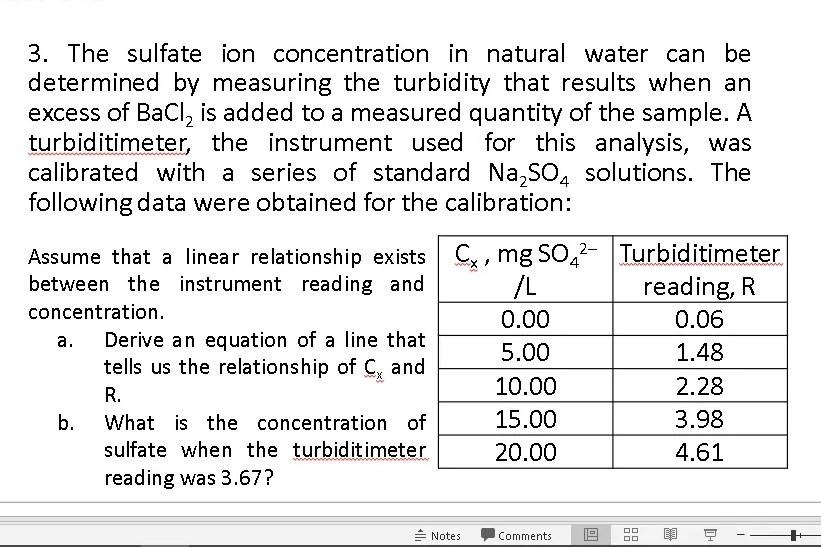
3. The sulfate ion concentration in natural water can be determined by measuring the turbidity that results when an excess of BaCl2 is added to a measured quantity of the sample. A turbiditimeter, the instrument used for this analysis, was calibrated with a series of standard Na2SO4 solutions. The following data were obtained for the calibration: a. Assume that a linear relationship exists Cx, mg SO42- Turbiditimeter between the instrument reading and /L reading, R concentration. 0.00 0.06 Derive an equation of a line that 5.00 1.48 tells us the relationship of Cy and R. 10.00 2.28 b. What is the concentration of 15.00 3.98 sulfate when the turbidit imeter 20.00 4.61 reading was 3.67? Notes Comments DI DD DO
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


