Question
Using your data from Table 2; rank the four metals Cu, Zn, Mg and Pb from the strongest reducing agent to the weakest reducing agent.
Using your data from Table 2; rank the four metals Cu, Zn, Mg and Pb from the strongest reducing agent to the weakest reducing agent. Recall that by "strong reducing agent" we mean it causes another metal to be reduced. (As opposed to itself undergoing reduction).
4. Using your data from Table 2; rank the four metal ions Cu2+, Zn2+, Mg2+ and Pb2+ from the strongest oxidizing agent to the weakest oxidizing agent . Recall that by "strong oxidizing agent" we mean it causes another metal to be oxidized. (As opposed to itself undergoing oxidation).
5. Data for reacting metallic Ag and metallic Cu with Ag(NO3)2 and Cu(NO3)2 is provided for you in Table 3. Using this data, where does silver fit in your ranking of the strongest reducing agents? Where does the silver ion fit in your ranking of the strongest oxidizing agents
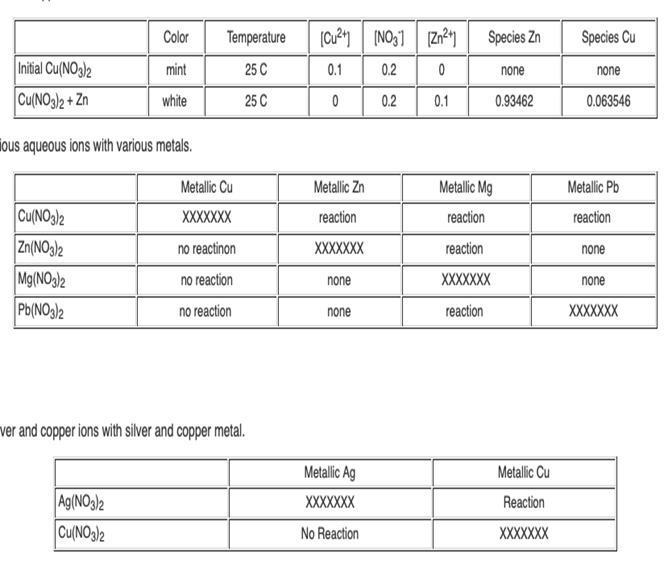
Color Temperature Species Zn Species Cu Initial Cu(NO3)2 mint 25 C 0.1 0.2 none none Cu(NO3)2 + Zn white 25 C 0.2 0.1 0.93462 0.063546 ious aqueous ions with various metals. Metallic Cu Metallic Zn Metallic Mg Metallic Pb Cu(NO3)2 Zn(NO3)2 Mg(NO3)2 Pb(NO3)2 XXXXXXX reaction reaction reaction no reactinon XXXXXXX reaction none no reaction none XXXXXXX none no reaction none reaction XXXXXXX ver and copper ions with silver and copper metal. Metallic Ag Metallic Cu Ag(NO3)2 Cu(NO3)2 XXXXXX Reaction No Reaction XXXXXXX
Step by Step Solution
3.48 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
Based on the observation given in the table the order of red...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


