Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. WHO AM I: Supposed the metal cation M** formed a paramagnetic tetrahedral complex [MJ4ly , having four unpaired electrons, with the monodentate anionic ligand
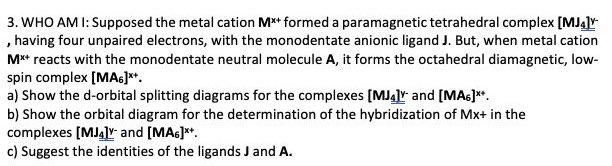
3. WHO AM I: Supposed the metal cation M** formed a paramagnetic tetrahedral complex [MJ4ly , having four unpaired electrons, with the monodentate anionic ligand J. But, when metal cation M** reacts with the monodentate neutral molecule A, it forms the octahedral diamagnetic, low- spin complex (MA)** a) Show the d-orbital splitting diagrams for the complexes [MJ4] and [MAs)**. b) Show the orbital diagram for the determination of the hybridization of Mx+ in the complexes [MJ4] and [MA6]** c) Suggest the identities of the ligands J and A
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


