
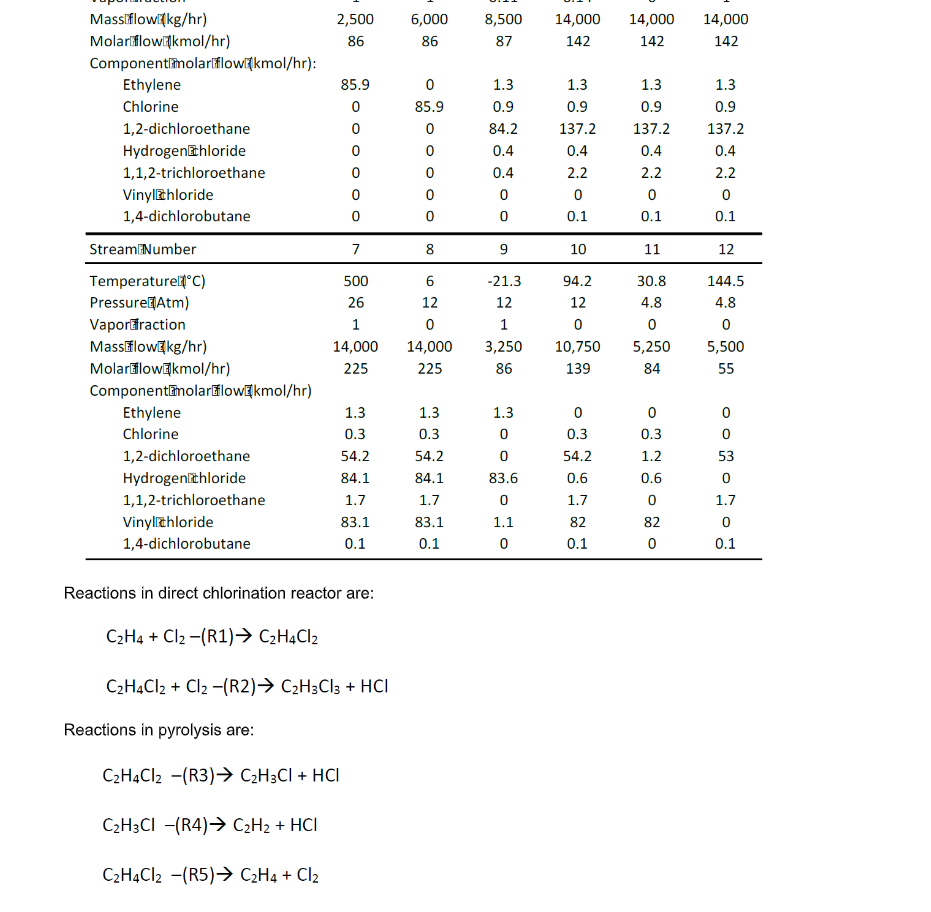

3.0 DESCRIPTION Ethylene and chlorine (streams 1 and 2) are fed in stoichiometric amounts at 25C and 1 atm to a 1.5 atm bubble column reactor (R-100) that circulates the reactant gases via sparger dispersion through liquid 1,2-dichloroethane that is maintained at 90C by a cold water stream (E- 100). The bubble column reactor design maximizes ethylene mass transfer and its use in industry is well-characterized (Orejas, 2001). The product stream exiting the direct chlorination reactor is a 97% pure 1,2-dichloroethane stream (stream 3) with limited side reaction and unreacted feed compounds. R-100 also contains ferric chloride that acts as a Lewis acid catalyst, polarizing chlorine to increase its electrophilic activity and encourage attack on ethylene's double bond (Wachi & Asai, Kinetics of 1,2-Dichloroethane Formation from Ethylene and Cupric Chloride, 1994). The 1,2-dichloroethane stream is joined by a recycle (producing stream 4) and fed through a centrifugal pump (P-100; stream 5) and a fired-heat evaporator (E-101; stream 6) to achieve pressurization to 26 atm, complete vaporization, and a temperature increase to 500C. This stream is flown into a pyrolysis furnace at the same temperature and pressure, where it is primarily converted to vinyl chloride. Although a catalytic pyrolysis method exists, similar conversion levels can be reached without a catalyst, making the non-catalytic approach a simpler, less expensive, and more favorable method (Dreher, Torkelson, & Beutel, 2012; Rus, 2013). The furnace contents are transferred to a quench tank (V-100; stream 7), which is maintained at 6C and 12 atm by circulating the liquid condensate exiting the pyrolysis furnace through a refrigerant- cooled condenser system (P-101, E-102). The quench tank is a vital component of this process: Without rapid cooling the pyrolysis reaction will continue, degrading desirable vinyl chloride and producing additional unwanted side products. The quench contents are fed to a 7-stage distillation column above stage 2 at 12 atm (T- 100; stream 8) where HCl is recovered at 97% purity in the distillate (stream 9) and the remainder of the pyrolysis products are fed in the bottoms stream to a second, similar, 9-stage distillation column above stage 3 at 4.8 atm (T-101; stream 10). This column yields a 97.6% pure vinyl chloride stream in the distillate (stream 11) and the 1,2-dichloroethane-rich bottoms stream is recycled ahead of the centrifugal pump (stream 12). The EFD and PFD for direct chlorination process is given in Figure 1 and 2, respectively. Summaries for all the process is given in Table 1. 2,500 86 6,000 86 8,500 87 14,000 142 14,000 142 14,000 142 1.3 1.3 1.3 1.3 85.9 0 0 0 0 0 0 0 85.9 0 0 0 0 0 0.9 84.2 0.4 0.4 0 0 0.9 137.2 0.4 2.2 0 0.1 0.9 137.2 0.4 2.2 0 0.1 0.9 137.2 0.4 2.2 0 0.1 7 8 9 10 11 12 Massiflowixkg/hr) Molartflowkmol/hr) Componentlmolarflowinkmol/hr): Ethylene Chlorine 1,2-dichloroethane Hydrogen&hloride 1,1,2-trichloroethane Vinyl chloride 1,4-dichlorobutane StreamNumber Temperaturelic) Pressure Atm) Vaporraction MassFlowEkg/hr) Molarflow&kmol/hr) ComponentlmolarflowEkmol/hr) Ethylene Chlorine 1,2-dichloroethane Hydrogenlithloride 1,1,2-trichloroethane Vinylzhloride 1,4-dichlorobutane 500 26 1 14,000 225 6 12 0 14,000 225 -21.3 12 1 3,250 86 94.2 12 0 10,750 139 30.8 4.8 0 5,250 84 144.5 4.8 0 5,500 55 1.3 0.3 54.2 84.1 1.7 83.1 0.1 1.3 0.3 54.2 84.1 1.7 83.1 0.1 1.3 0 0 83.6 0 1.1 0 0 0.3 54.2 0.6 1.7 82 0.1 0 0.3 1.2 0.6 0 0 53 0 1.7 0 0.1 0 82 0 Reactions in direct chlorination reactor are: C2H4 + Cl2-(R1) C2H4Cl2 C2H4Cl2 + Cl2 -(R2) C2H3Cl3 + HCI Reactions in pyrolysis are: C2H4Cl2 -(R3) C2H3Cl + HCI C2H3CI -(R4) C2H2 + HCI C2H4Cl2 -(R5) C2H4 + Cl2 4.0 YOUR TASK Evaluate on the heat required by one (1) reactive process and one (1) non-reactive process. List all data (such as heat capacity or heat of formation of all components/compounds) and assumptions used to help you with the evaluation and provide the references (whenever possible). Also, derive a differential balance on total mass and vinyl chloride if Stream 11 is to be stored in a well-mixed tank. Provide your initial conditions. Predict volume of the tank to be installed if the tank requires 8 hours to be fully filled









