Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(30 pts.) The following irreversible second order gas phase reaction is run in a CSTR equipped with a heat exchanger. AB The composition of
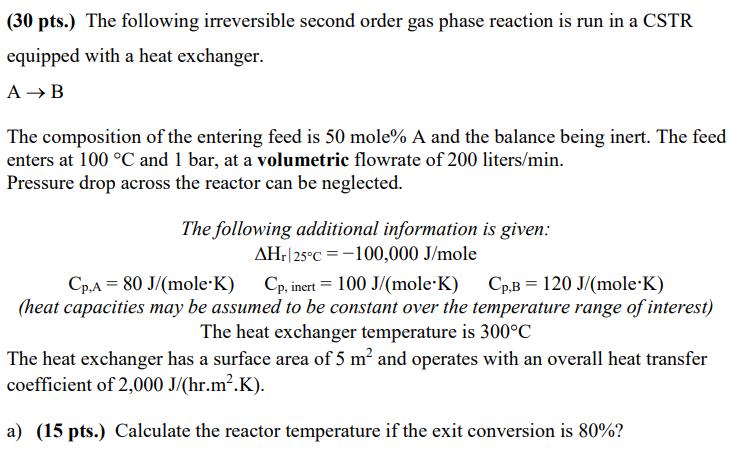

(30 pts.) The following irreversible second order gas phase reaction is run in a CSTR equipped with a heat exchanger. AB The composition of the entering feed is 50 mole% A and the balance being inert. The feed enters at 100 C and 1 bar, at a volumetric flowrate of 200 liters/min. Pressure drop across the reactor can be neglected. The following additional information is given: AHr| 25C -100,000 J/mole Cp.A 80 J/(mole K) Cp, inert = 100 J/(mole*K) Cp.B 120 J/(mole K) (heat capacities may be assumed to be constant over the temperature range of interest) The heat exchanger temperature is 300C The heat exchanger has a surface area of 5 m and operates with an overall heat transfer coefficient of 2,000 J/(hr.m.K). a) (15 pts.) Calculate the reactor temperature if the exit conversion is 80%? b) (15 pts.) Calculate the reaction rate constant given that the reactor volume is equal to 500 liters (Use conversion from part a).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


