\\

*3.15 is just provided for reference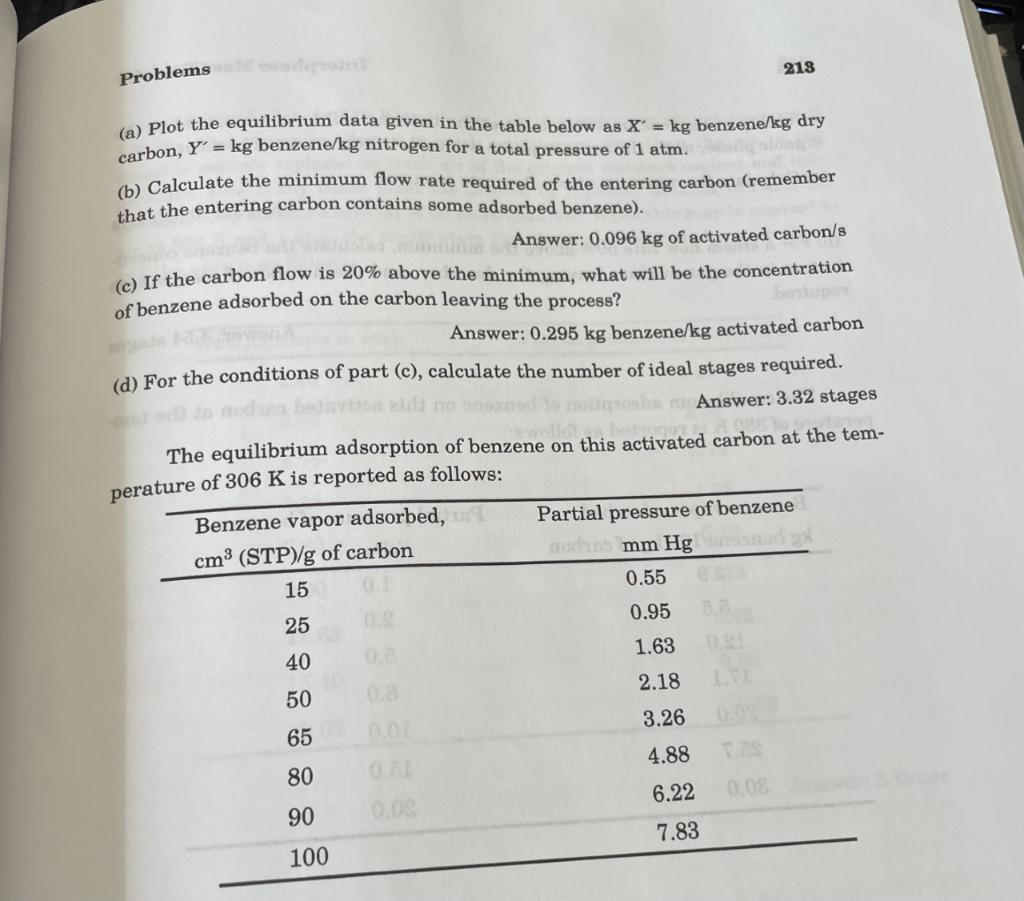
8.17b. Material balancess adsorptlon of bennene vapor on activated carbon; cocurrent operation If the adsorption process described in Problem 3.15 took place cocurrently, calculate the minimum flow rate of activated carbon required. Answer: 0.543kg/s 3.15. Material balances: adsorption of benzene vapor on activated Activated carbon is used to recover benzene from a nitrogen-benzene vapor carbon mixture. The mixture, at 306K and 1atm containing 1.0% benzene by volume, is to be passed countercurrently at the rate of 1.0m3/s to a moving stream of activated carbon so as to remove 85% of the benzene from the gas in a continuous process. The entering activated carbon contains 15cm3 of benzene vapor (at STP) adsorbed per gram of the carbon. The temperature and total pressure are maintained at 306K and 1atm. Nitrogen is not adsorbed. (a) Plot the equilibrium data given in the table below as X=kg benzene/kg dry carbon, Y=kg benzene/ kg nitrogen for a total pressure of 1atm. (b) Calculate the minimum flow rate required of the entering carbon (remember that the entering carbon contains some adsorbed benzene). Answer: 0.096kg of activated carbon/s (c) If the carbon flow is 20% above the minimum, what will be the concentration of benzene adsorbed on the carbon leaving the process? Answer: 0.295kg benzene/kg activated carbon (d) For the conditions of part (c), calculate the number of ideal stages required. Answer: 3.32 stages The equilibrium adsorption of benzene on this activated carbon the temof 306K is reported as follows: 8.17b. Material balancess adsorptlon of bennene vapor on activated carbon; cocurrent operation If the adsorption process described in Problem 3.15 took place cocurrently, calculate the minimum flow rate of activated carbon required. Answer: 0.543kg/s 3.15. Material balances: adsorption of benzene vapor on activated Activated carbon is used to recover benzene from a nitrogen-benzene vapor carbon mixture. The mixture, at 306K and 1atm containing 1.0% benzene by volume, is to be passed countercurrently at the rate of 1.0m3/s to a moving stream of activated carbon so as to remove 85% of the benzene from the gas in a continuous process. The entering activated carbon contains 15cm3 of benzene vapor (at STP) adsorbed per gram of the carbon. The temperature and total pressure are maintained at 306K and 1atm. Nitrogen is not adsorbed. (a) Plot the equilibrium data given in the table below as X=kg benzene/kg dry carbon, Y=kg benzene/ kg nitrogen for a total pressure of 1atm. (b) Calculate the minimum flow rate required of the entering carbon (remember that the entering carbon contains some adsorbed benzene). Answer: 0.096kg of activated carbon/s (c) If the carbon flow is 20% above the minimum, what will be the concentration of benzene adsorbed on the carbon leaving the process? Answer: 0.295kg benzene/kg activated carbon (d) For the conditions of part (c), calculate the number of ideal stages required. Answer: 3.32 stages The equilibrium adsorption of benzene on this activated carbon the temof 306K is reported as follows









