Question
3.2 When the number of particles changes in a thermodynamic transformation, it is important to use the correct form of entropy for an ideal
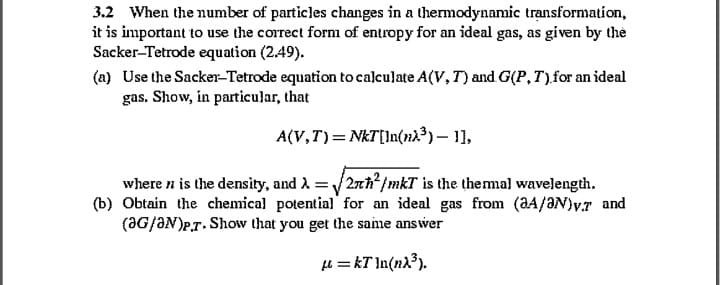
3.2 When the number of particles changes in a thermodynamic transformation, it is important to use the correct form of entropy for an ideal gas, as given by the Sacker-Tetrode equation (2.49). (a) Use the Sacker-Tetrode equation to calculate A(V, T) and G(P, T) for an ideal gas. Show, in particular, that A(V,T) = NkT[In(n)) 1], where it is the density, and =2h/mkT is the thermal wavelength. (b) Obtain the chemical potential for an ideal gas from (2A/3N) v.7 and (G/N)P.T. Show that you get the same answer =kT]n(n).
Step by Step Solution
3.49 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Solutions So we have fourtition function for idea Jas Soi Zn 2 2 Vmk...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Business Statistics A Decision Making Approach
Authors: David F. Groebner, Patrick W. Shannon, Phillip C. Fry
9th Edition
013302184X, 978-0133021844
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App