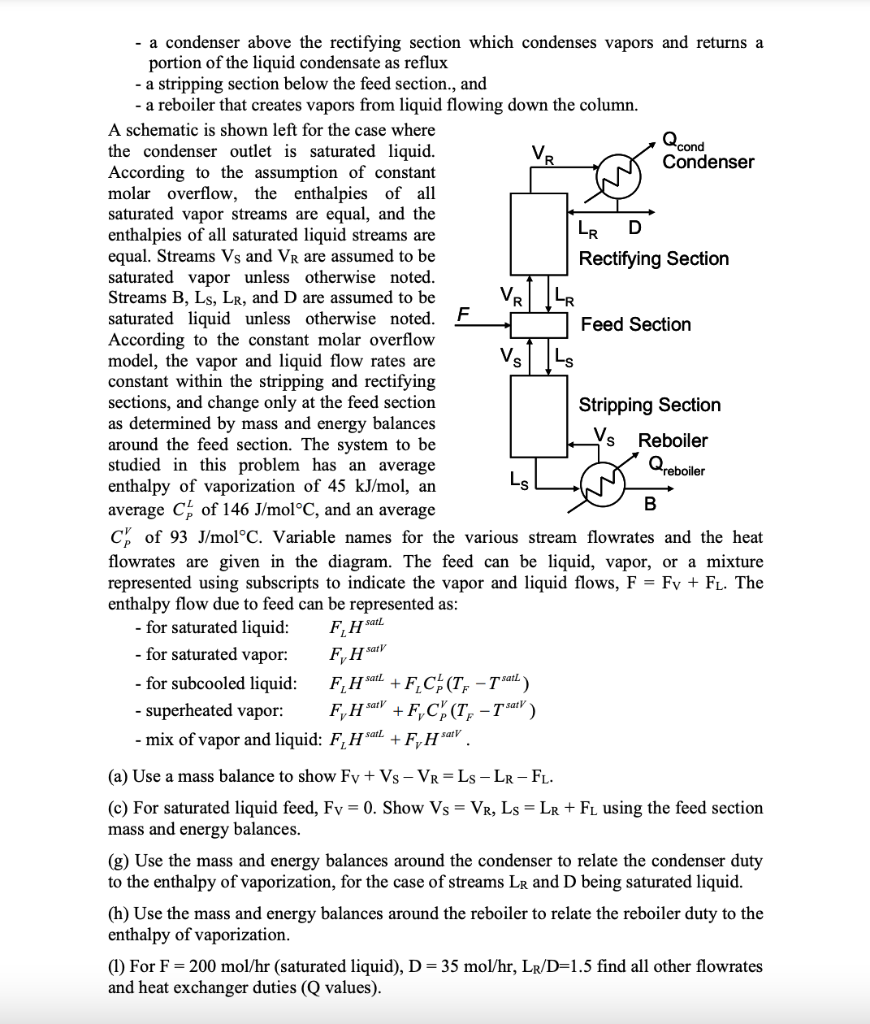
= + 3.3 A common model in distillation column screening is called constant molar overflow. In this model, the actual enthalpy of vaporization of a mixture is represented by the average enthalpy of vaporization, which can be assumed to be independent of composition for the purpose of calculation, AH vap = (AH yap + AH yap)/2. A distillation column operates by contacting concurrent streams of vapor and liquid using internal trays or packing. The actual design the trays or packing is not required to perform an energy balance using the constant molar overflow concepts. In the constant molar overflow model for a column with one feed, the column may be represented by 5 sections: - a feed section where the feed enters - a rectifying section above the feed zone Qcond - a condenser above the rectifying section which condenses vapors and returns a portion of the liquid condensate as reflux - a stripping section below the feed section., and - a reboiler that creates vapors from liquid flowing down the column. A schematic is shown left for the case where the condenser outlet is saturated liquid. Condenser According to the assumption of constant molar overflow, the enthalpies of all saturated vapor streams are equal, and the enthalpies of all saturated liquid streams are LR D equal. Streams Vs and VR are assumed to be Rectifying Section saturated vapor unless otherwise noted. Streams B, Ls, LR, and D are assumed to be VR. saturated liquid unless otherwise noted. F Feed Section According to the constant molar overflow model, the vapor and liquid flow rates are V. constant within the stripping and rectifying sections, and change only at the feed section Stripping Section as determined by mass and energy balances around the feed section. The system to be Vs Reboiler studied in this problem has an average enthalpy of vaporization of 45 kJ/mol, an Ls B average C, of 146 J/molC, and an average C of 93 J/molC. Variable names for the various stream flowrates and the heat flowrates are given in the diagram. The feed can be liquid, vapor, or a mixture represented using subscripts to indicate the vapor and liquid flows, F = Fy + Fl. The enthalpy flow due to feed can be represented as: - for saturated liquid: - for saturated vapor: - for subcooled liquid: F, H sal + F C (T; - 1 sat) - superheated vapor: +F,C%(T; - Tsair) - mix of vapor and liquid: F H sal + F H satv. Qreboiler F, HAL F, HSV F, Sarv (a) Use a mass balance to show Fy + Vs - VR=Ls - LR-FL. (c) For saturated liquid feed, Fy = 0. Show Vs = VR, Ls = LR + F using the feed section mass and energy balances. (g) Use the mass and energy balances around the condenser to relate the condenser duty to the enthalpy of vaporization, for the case of streams LR and D being saturated liquid. (h) Use the mass and energy balances around the reboiler to relate the reboiler duty to the enthalpy of vaporization. (1) For F= 200 mol/hr (saturated liquid), D= 35 mol/hr, Lr/D=1.5 find all other flowrates and heat exchanger duties (Q values)








