Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3.5. Look up the radii of Ti4+, Ba+, and O- listed in App. 3A, and making use of Pauling's size criteria, choose the most
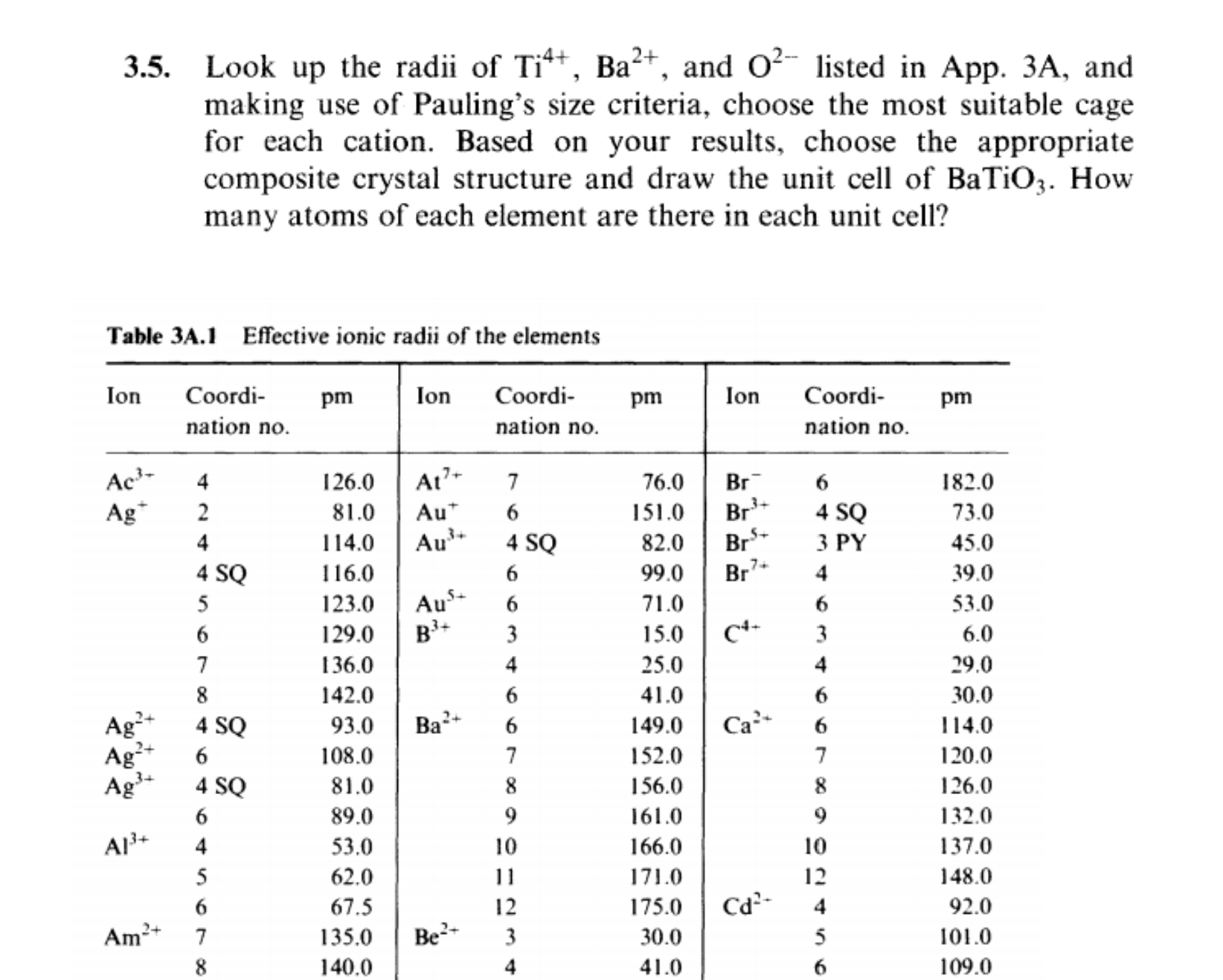
3.5. Look up the radii of Ti4+, Ba+, and O- listed in App. 3A, and making use of Pauling's size criteria, choose the most suitable cage for each cation. Based on your results, choose the appropriate composite crystal structure and draw the unit cell of BaTiO3. How many atoms of each element are there in each unit cell? Table 3A.1 Effective ionic radii of the elements Ion Coordi- pm nation no. Ac- Ag+ Ag+ Ag+ Ag 4 SQ 6 A1+ 4 Am 4 2 4 4 SQ 5 6 7 8 4 SQ 2+ 5 6 7 8 Ion At7+ Au Au+ Coordi- nation no. 126.0 81.0 114.0 116.0 123.0 129.0 136.0 142.0 93.0 108.0 81.0 89.0 53.0 62.0 67.5 12 135.0 Be+ 3 140.0 4 7 6 4 SQ 6 Ba+ Aus+ 6 B+ 3 4 6 6 7 8 9 10 pm Ion 76.0 Br 151.0 Br 82.0 Br+ 99.0 Br 71.0 15.0 25.0 41.0 149.0 152.0 156.0 161.0 166.0 171.0 175.0 30.0 41.0 C4+ Ca+ Cd- Coordi- nation no. 6 4 SQ 3 PY] 4 4 6 7 8 9 10 22456 12 pm 182.0 73.0 45.0 39.0 53.0 6.0 29.0 30.0 114.0 120.0 126.0 132.0 137.0 148.0 92.0 101.0 109.0
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


