Answered step by step
Verified Expert Solution
Question
1 Approved Answer
37. The forecast for the monthly demand of a product is given in the table below. Month Forecast Actual Sales 1 32.00 30.00 2
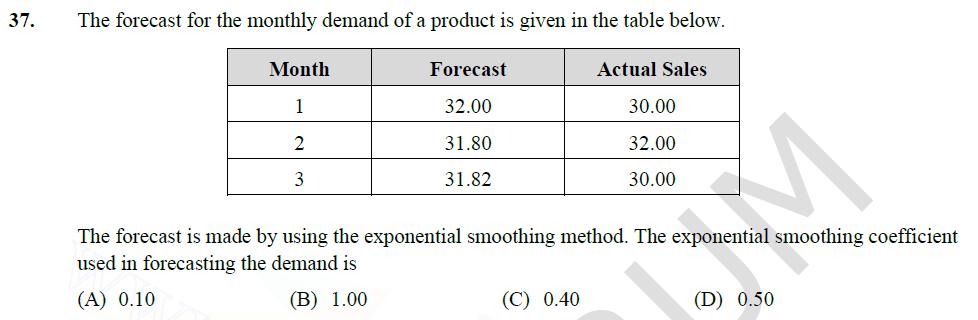
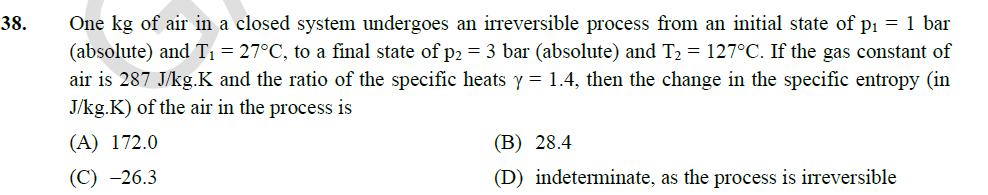

37. The forecast for the monthly demand of a product is given in the table below. Month Forecast Actual Sales 1 32.00 30.00 2 31.80 32.00 3 31.82 30.00 The forecast is made by using the exponential smoothing method. The exponential smoothing coefficient used in forecasting the demand is (A) 0.10 (B) 1.00 (C) 0.40 (D) 0.50 38. One kg of air in a closed system undergoes an irreversible process from an initial state of p = 1 bar (absolute) and T = 27C, to a final state of p2 = 3 bar (absolute) and T2 = 127C. If the gas constant of air is 287 J/kg.K and the ratio of the specific heats y = 1.4, then the change in the specific entropy (in J/kg.K) of the air in the process is (A) 172.0 (C) -26.3 (B) 28.4 (D) indeterminate, as the process is irreversible /2 39. For the integral (8+4 cosx)dx, the absolute percentage error in numerical evaluation with the (round off to one decimal place). Trapezoidal rule, using only the end points, is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


