Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3.A The rale constant of a chemical reaction increased from 0.100s1t03.10s1 upon raising the Learning Goal: temperature from 250C to 4900C. To use the Adthenius
3.A 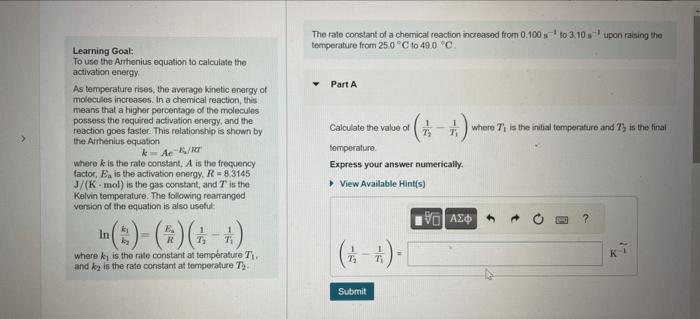
The rale constant of a chemical reaction increased from 0.100s1t03.10s1 upon raising the Learning Goal: temperature from 250C to 4900C. To use the Adthenius equation to calculate the activation energy. As temperature rises, the avorage kinetic enargy of Part A molecules increases. In a chemical reaction, this means that a higher percentage of the molecules possess the required activation energy, and the reaction goes faster. This relationship is shown by the Arsthenius equation k=AeL2/RI Calculate the value of (T21T11) where T1 is the initial tempenture and T2 is the final where k is the rate constant, A is the freguency factor, Fa is the activation energy. R=8.3145 J/(K+mol) is the gas oonstant, and T is the Kelvin temperature. The following reartangod versicn of the equation is also useful: ln(k2k1)=(REa)(T11T11) femperature. Express your answer numericallyf where k1 is the rate constant at temperature Tr. and k2 is the rate constant at temperature T2 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


