Answered step by step
Verified Expert Solution
Question
1 Approved Answer
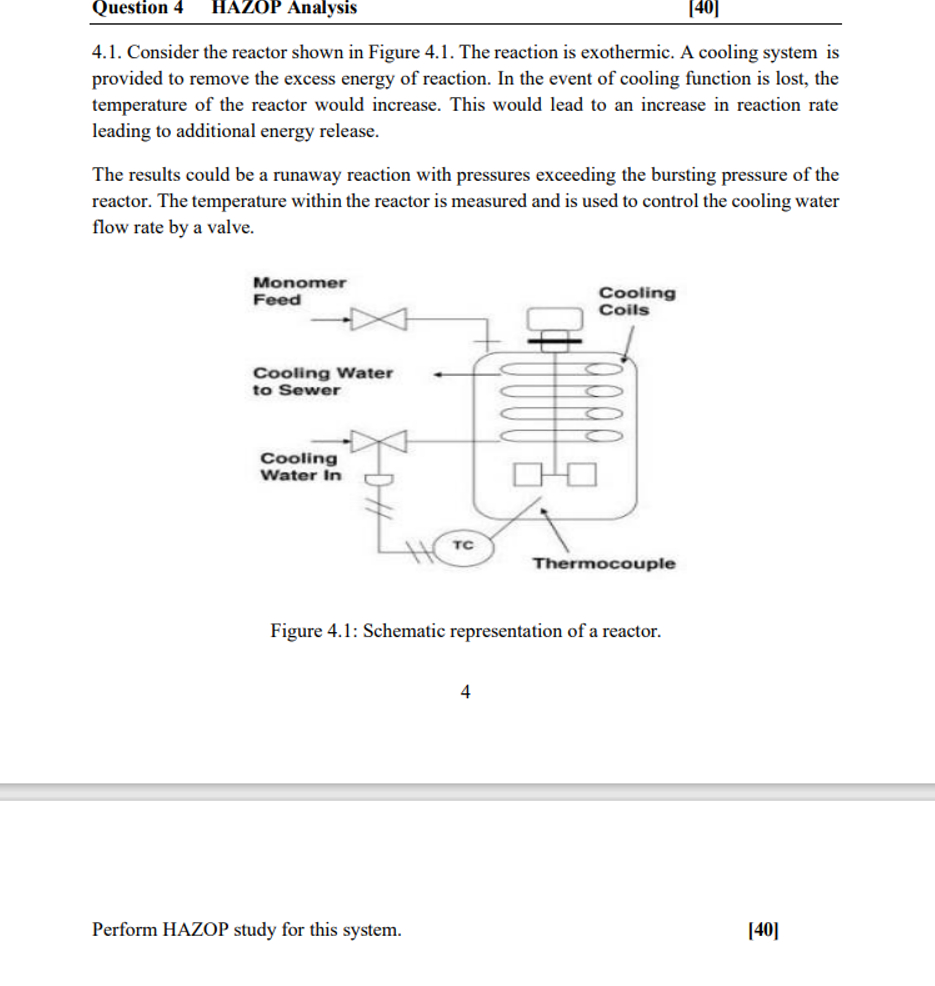
4 . 1 . Consider the reactor shown in Figure 4 . 1 . The reaction is exothermic. A cooling system is provided to remove
Consider the reactor shown in Figure The reaction is exothermic. A cooling system is
provided to remove the excess energy of reaction. In the event of cooling function is lost, the
temperature of the reactor would increase. This would lead to an increase in reaction rate
leading to additional energy release.
The results could be a runaway reaction with pressures exceeding the bursting pressure of the
reactor. The temperature within the reactor is measured and is used to control the cooling water
flow rate by a valve.
Figure : Schematic representation of a reactor.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


