Answered step by step
Verified Expert Solution
Question
1 Approved Answer
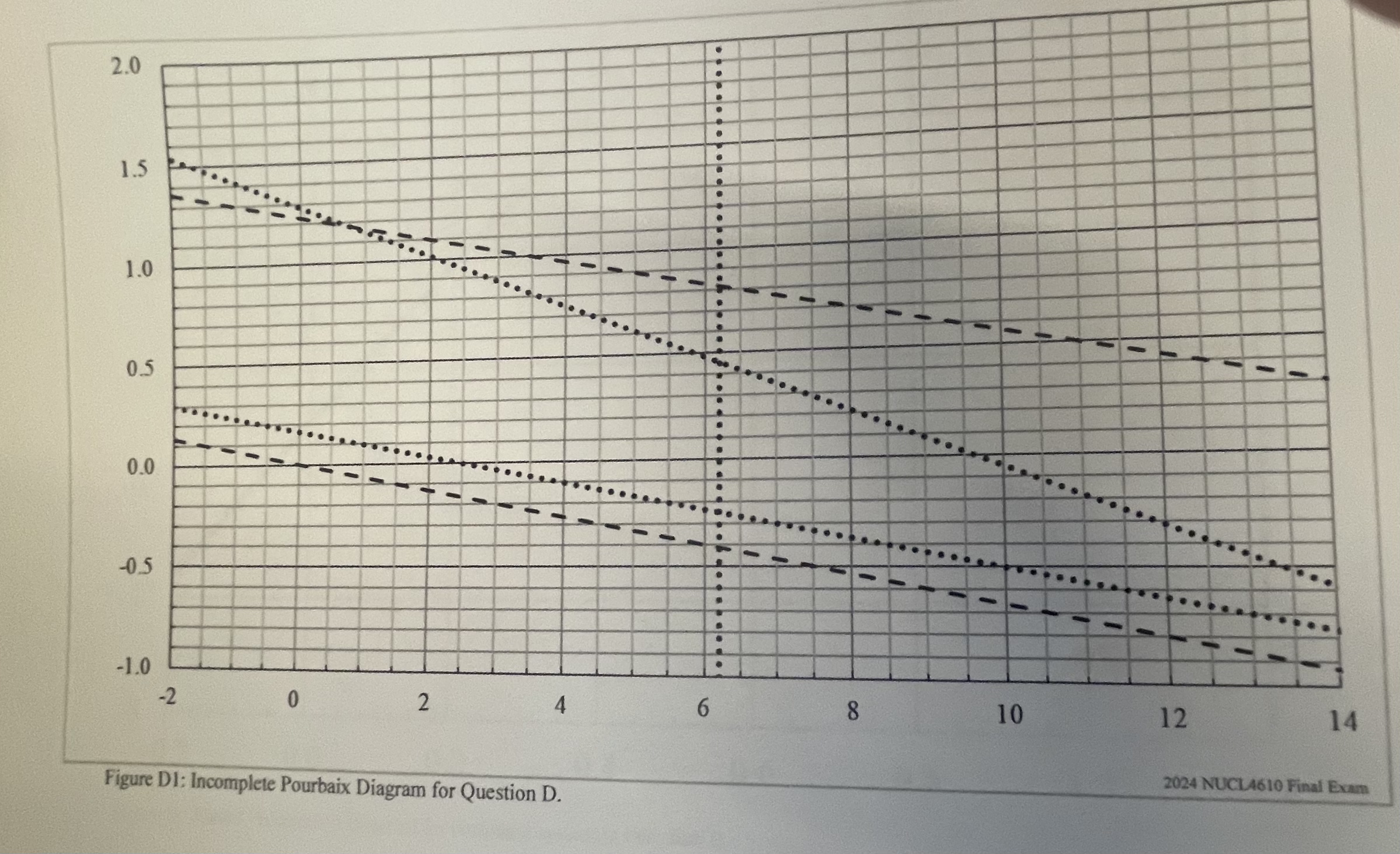
4 3 Marks Section D . Thermodynamics and Pourbaix Diagrams Your lab is measuring the thermchemical properties of Chronius - 8 9 ( C 8
Marks
Section D Thermodynamics and Pourbaix Diagrams
Your lab is measuring the thermchemical properties of ChroniusC a new formulation of an intermetallic composite that behaves like a single element designated The intended use of the alloy is a lightweight, highpurity water tank for storing purified water. You team has been working for a to develop appropriate thermochemical data and has finally demonstrated the system works at the maximum design temperature, This is the temperature of the condensate from the final distillation column for ultimate purification. The Principal Investigator has just been escorted off site, leaving you to finish the Pourbaix diagram and writeup the report. The final processed data is provided in Table D A partial Pourbaix diagram is shown in Figure Dend of exam but unfortunately it is not labeled and only shows some sketched lines dotted lines
Note: at
Table D: Equilibrium Data for in Pure Water at
tableReaction
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


