Answered step by step
Verified Expert Solution
Question
1 Approved Answer
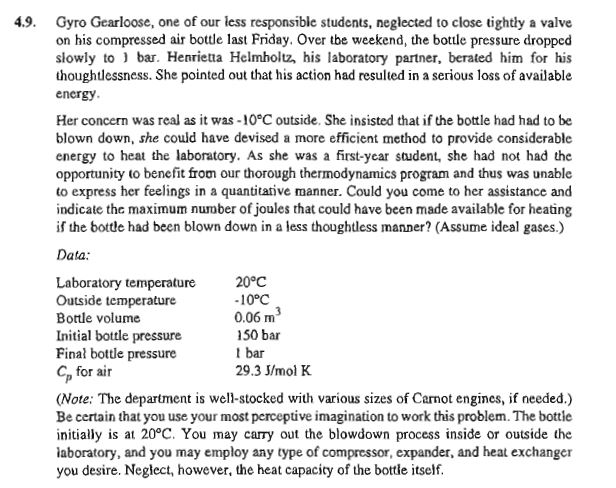
4 . 9 . Gyro Gearloose, one of our less responsibie students, neglected to close tightly a valve on his compressed air bottle last Friday.
Gyro Gearloose, one of our less responsibie students, neglected to close tightly a valve
on his compressed air bottle last Friday. Over the weekend, the bottle pressure dropped
slowly to bar. Henrietta Helmholtz, his laboratory partner, berated him for his
thoughtlessness. She pointed out that his action had resulted in a serious loss of available
energy.
Her concern was real as it was outside. She insisted that if the bottle had had to be
blown down, she could have devised a more efficient method to provide considerable
energy to heat the laboratory. As she was a firstyear student, she had not had the
opportunity to benefit from our thorough thermodynamics program and thus was unable
to express her feelings in a quantitarive manner. Could you come to her assistance and
indicate the maximum number of joules that could have been made available for heating
if the bottle had been blown down in a less thoughtless manner? Assume ideal gases.
Data:
Note: The depariment is wellstocked with various sizes of Carnot engines, if needed.
Be certain that you use your most perceptive imagination to work this problem. The bottie
initially is at You may carry out the blowdown process inside or outside the
iaboratory, and you may employ any type of compressor, expander, and heat exchanger
you desire. Neglect, however, the heat capacity of the bottle itself.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


