Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4) A 0.04% carbon steel (low alloy steel) is produced in an electrical arc furnace at 1600 C. The oxygen content of the steel is
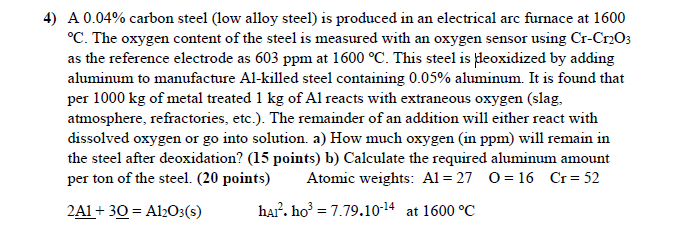 4) A 0.04% carbon steel (low alloy steel) is produced in an electrical arc furnace at 1600 C. The oxygen content of the steel is measured with an oxygen sensor using CrCr2O3 as the reference electrode as 603ppm at 1600C. This steel is pleoxidized by adding aluminum to manufacture Al-killed steel containing 0.05% aluminum. It is found that per 1000kg of metal treated 1kg of Al reacts with extraneous oxygen (slag, atmosphere, refractories, etc.). The remainder of an addition will either react with dissolved oxygen or go into solution. a) How much oxygen (in ppm) will remain in the steel after deoxidation? (15 points) b) Calculate the required aluminum amount per ton of the steel. (20 points) Atomic weights: Al=27O=16Cr=52 2Al+3O=Al2O3(s)hAl2ho3=7.791014at1600C
4) A 0.04% carbon steel (low alloy steel) is produced in an electrical arc furnace at 1600 C. The oxygen content of the steel is measured with an oxygen sensor using CrCr2O3 as the reference electrode as 603ppm at 1600C. This steel is pleoxidized by adding aluminum to manufacture Al-killed steel containing 0.05% aluminum. It is found that per 1000kg of metal treated 1kg of Al reacts with extraneous oxygen (slag, atmosphere, refractories, etc.). The remainder of an addition will either react with dissolved oxygen or go into solution. a) How much oxygen (in ppm) will remain in the steel after deoxidation? (15 points) b) Calculate the required aluminum amount per ton of the steel. (20 points) Atomic weights: Al=27O=16Cr=52 2Al+3O=Al2O3(s)hAl2ho3=7.791014at1600C Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


